I n 1880, Jules Cotard (1840–1889) described the syndrome that bears his name as a constellation of false nihilistic beliefs, often in the form of self-negation. Cotard was an eminent French physician dedicated to both neurological and psychiatric patients.
1 Although there is historical controversy over the precise clinical picture of Cotard syndrome, it is most often an eponym for
deliré des negations, translated to English as “nihilistic delusion.” The original French term
deliré, however, is wider than the English term “delusion” and may include intellectual, emotional, and volitional symptoms, which is why some authors prefer the term “Cotard syndrome.”
2 Some authors have equated Cotard syndrome with the delusion of being dead,
3 –
6 but this is not essential to the syndrome. According to a historical statistical analysis, the denial of self-existence is present in only 69% of the cases, while, paradoxically, 55% of the patients may show delusions of immortality.
7 There are several proposed mechanisms for Cotard syndrome. It may begin with anomalous perceptual experiences or their disconnection from emotional or limbic processes, and there may be a failure in belief evaluation or a tendency to negative self-attribution.
3,
5,
6,
8 Many patients have psychiatric disease with psychotic depression, an internalized attributional style, or associated depersonalization.
2,
5 Others have neurological disease with Parkinson’s disease, migraine, brain tumors, traumatic brain injury, arteriovenous malformations, and multiple sclerosis.
9,
10 Any understanding of Cotard syndrome needs to account for both neurological and psychiatric patients.
This aim of this article was to survey the prevalence of Cotard syndrome in a large neuropsychiatric institution and to describe the clinical expressions, imaging data, treatment, and potential psychobiological mechanisms for this fascinating syndrome that traverses the boundary between neurological and psychiatric disease.
CASE REPORTS
Patient 1
A previously healthy 18-year-old woman was hospitalized after sudden generalized tonic-clonic seizures. Phenytoin controlled her seizures, but she remained disoriented with psychomotor agitation, motiveless crying, and auditory hallucinations. She also repeatedly complained about missing both of her hands, prompting a neuropsychiatric consultation for “asomatognosia.” Her neurological examination was otherwise normal except for generalized hyperreflexia.
Her MRI did not show structural abnormalities. The EEG had generalized dysfunction without epileptic activity. Her lumbar puncture was normal except for an increase in leukocytes at 17 per mm 3 . A search for bacteria, fungi, parasites, and DNA viruses (herpes simplex, cytomegalovirus, varicella-zoster, and Epstein-Barr) was negative, so a diagnosis of probable nonherpetic viral encephalitis was made.
After 3 weeks, she gradually recovered from the acute encephalopathy and became alert, fluent, and coherent in her verbal output. Nevertheless, over several days, she continued with Cotard-type self-negation: “I am sick because they haunted me, my heart stopped working, and I feel that my liver and my stomach are getting sick, they stopped working; I do not feel my body from the inside. I have no heart.” On several occasions, she expressed the belief that she did not exist because she could not feel herself. A restricted affect with alexithymia completed the clinical picture, but there were no depressive features. Cotard features remained for another week until discharge but diminished significantly with the use of risperidone. Three weeks later, as an outpatient, she had a complete remission of nihilistic delusions.
Patient 2
A 48-year-old male musician was hospitalized for severe anxiety and depression. He had been well until approximately 1 month prior to admission when his family found him anxious, sad, and feeling guilty because of “many sins.” He paced back and forth, anxiously pulling his hair, and then experienced auditory hallucinations but refused to relate their content. Five days later he complained of not feeling as if he was himself. On admission, he expressed delusions of reference and harm but he also repeated that nothing was true: “The hospitals do not exist, the doctors do not exist… I’m scared.” He appeared perplexed and frightened, looking at his hands and touching his face repeatedly, and stated that nothing existed, including himself. Physical and neurologic examinations were otherwise normal. A computed tomography (CT) scan of the brain was unremarkable, and lumbar puncture revealed no cells and normal levels of proteins and glucose in the CSF.
The patient’s past history was significant for similar psychiatric symptoms, with episodes of depression and many episodes of depersonalization and derealization, without delusional or hallucinatory phenomena, which resolved spontaneously in the course of hours or days.
During the initial 24 hours, the patient refused to eat. He continuously stared at his hands, sometimes stating, “I do not have hands,” “Nothing exists,” “I feel guilty.” Clonazepam and risperidone were started, and amitryptiline was added later to his treatment. He gradually became alert, oriented, attentive, and cooperative but continued to feel guilty about his behavior. He was discharged after 12 days on amitryptiline (75 mg) and risperidone (2 mg). Two weeks later, on follow-up, the patient was working half-time singing and playing the guitar. His mood appeared normal although he continued to feel situational anxiety and guilt. He denied symptoms of depersonalization, derealization, delusions, or hallucinations.
Patient 3
A 78-year-old internist presented with a 7-month history of depressed mood, anhedonia, anorexia with a loss of 20 pounds over several weeks, loss of interest in self-care and hygiene, ideas of self-loathing, and delusional ideas of ruin and catastrophe. In the emergency ward, he rejected hospitalization with the argument that he was “already dead.” In the neuropsychiatric ward, he repeatedly stated, “I’m a terminal patient” and “I’m done. There’s no point in treating me. I’m terminal.” Later on he stated, “I am no longer myself, I fell like an automaton, like if the world did not exist; I am completely eliminated.” He also stated that “the food I eat sticks to my bowel, that′s why I don’t eat anymore, that’s why I don′t even drink water or take medications, and I don′t defecate because I have hemorrhoids.” Also he insisted that he had lost his right kidney and that the left kidney did not work anymore. His past medical history was remarkable for hypertension, treated with enalapril, 10 mg/day, and nifedipine, 30 mg/day; gout treated with allopurinol; the prior expulsion of a kidney stone; and benign prostatic hypertrophic prostate treated with transurethral resection.
His examination suggested parkinsonism. On gait examination, he had marked slowing in all of his movements with very small steps, a loss of associated arm movements, and a mild loss of postural reflexes with deviation of the trunk to his left. On cranial nerve examination, he had minimal facial expressivity resembling hypomimia, with a slow and low volume of speech. On motor examination, he had generalized bradykinesia and mild limb and axial rigidity but no tremors. On the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS), he scored 35 points, indicating mild to moderate severity. MRI showed an increase in the subarachnoid space in both the frontolateral areas and a mild dilation in the ventricular system.
The patient was diagnosed with major depressive episode with psychotic symptoms and with the suspicion of a rigid-akinetic syndrome (probably idiopathic Parkinson’s disease). He received mirtazapine, 60 mg/daily, and ECT, and after three sessions he had a marked improvement of his gait and speed and amplitude of movements. He also experienced a decrease in the motor part of the UPDRS to 23 points (34% decrease, which is considered a good response). After the eighth ECT session, there was an evident improvement of mood, decreased anxiety, and resolution of his Cotard-like beliefs. He was discharged after 31 days of hospitalization, and follow-up at 1 month showed absence of depression or a return of his parkinonian features.
Patient 4
A 55-year-old successful restauranteur was hospitalized with recurrent psychotic depression. After several economic problems, his brothers found him in bed for several weeks. The patient would not eat, stand up, or allow himself to be attended to. He just cried and admitted he could not sleep. He did not want to be hospitalized as he was sure that it was a plot to eliminate him. Later on he revealed to his family that his penis had shortened until it disappeared but refused to verify this problem in front of others.
On hospitalization, his physical and neurological examinations were normal, but he continued to express nihilistic beliefs. During ophthalmoscopy, he stated that “I have no eyes.” Even when he looked in the mirror, he insisted his eyes had been taken out by the emergency room physician. During an ECG, he affirmed that he had no heart. He did not want to hear its beat with the use of stethoscope, because he was convinced that his heart had been taken out by the same emergency room doctor. In addition, he insisted that his left hand was dead and, at times, would claim that he was already a dead person.
The rest of his examination included past psychiatric history, mental status examination, and laboratory tests. At age 51 the patient had had a major depressive episode with psychotic symptoms and was hospitalized for 1 month. He received medication for 6 weeks with complete remission. During the current admission, he had an initial Mini-Mental State Examination (MMSE) score of 19/30 and marked constructional problems on the Neurobehavioral Cognitive State Examination (COGNISTAT). His admission EEG and MRI were normal.
The patient was successfully treated with medications and ECT. He initially received 10 mg of olanzapine and 20 mg of citalopram, and he subsequently required ECT. After a course of ECT, his mood improved, and he admitted to severe depression. His delusional ideas disappeared, but he continued to be distrustful of any examination of his left hand. On discharge, his MMSE was 27/30, and his isolated problems in constructional abilities continued, which he claimed were lifelong. Outpatient follow-up confirmed continued remission on 20 mg of citalopram and 2.5 mg of olanzapine per day.
DISCUSSION
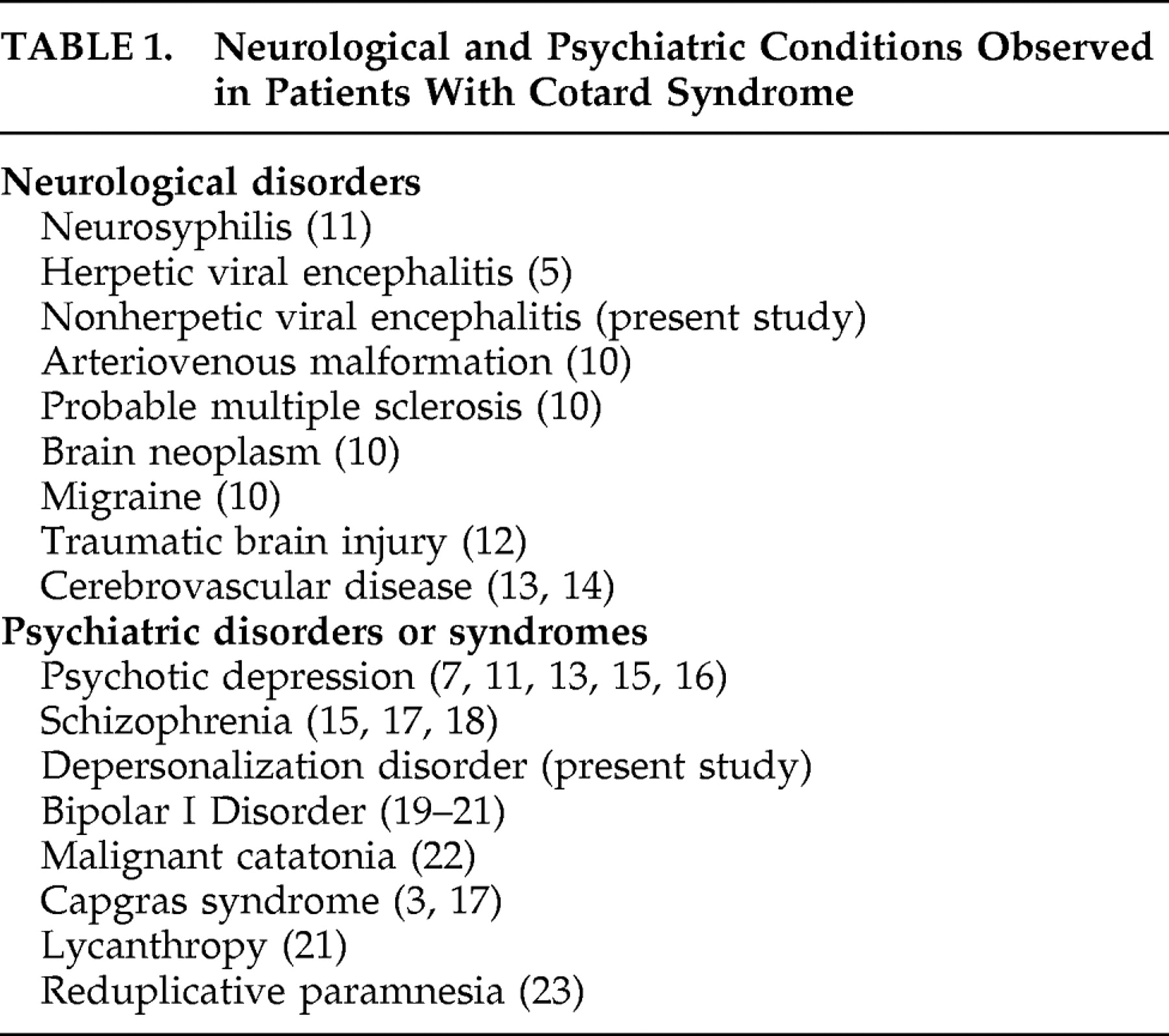
Cotard syndrome may be seen in both neurological and psychiatric patients, although its frequency is rare according to this study and considering the fact that previous studies are based on single cases and small case series. This study does not provide information on outpatients with neurological or psychiatric disease. A general limitation for systematic studies lies on the fact that there is a lack of clinical instruments or diagnostic criteria for the measurement of Cotard syndrome, so at this point it is based mainly in skilled psychiatric interview. Cotard syndrome can clearly result from a variety of neurological and psychiatric conditions (see
Table 1 ). In the patients we present, several psychopathological (and possibly neurobiological) mechanisms may explain the production of their nihilistic delusions.
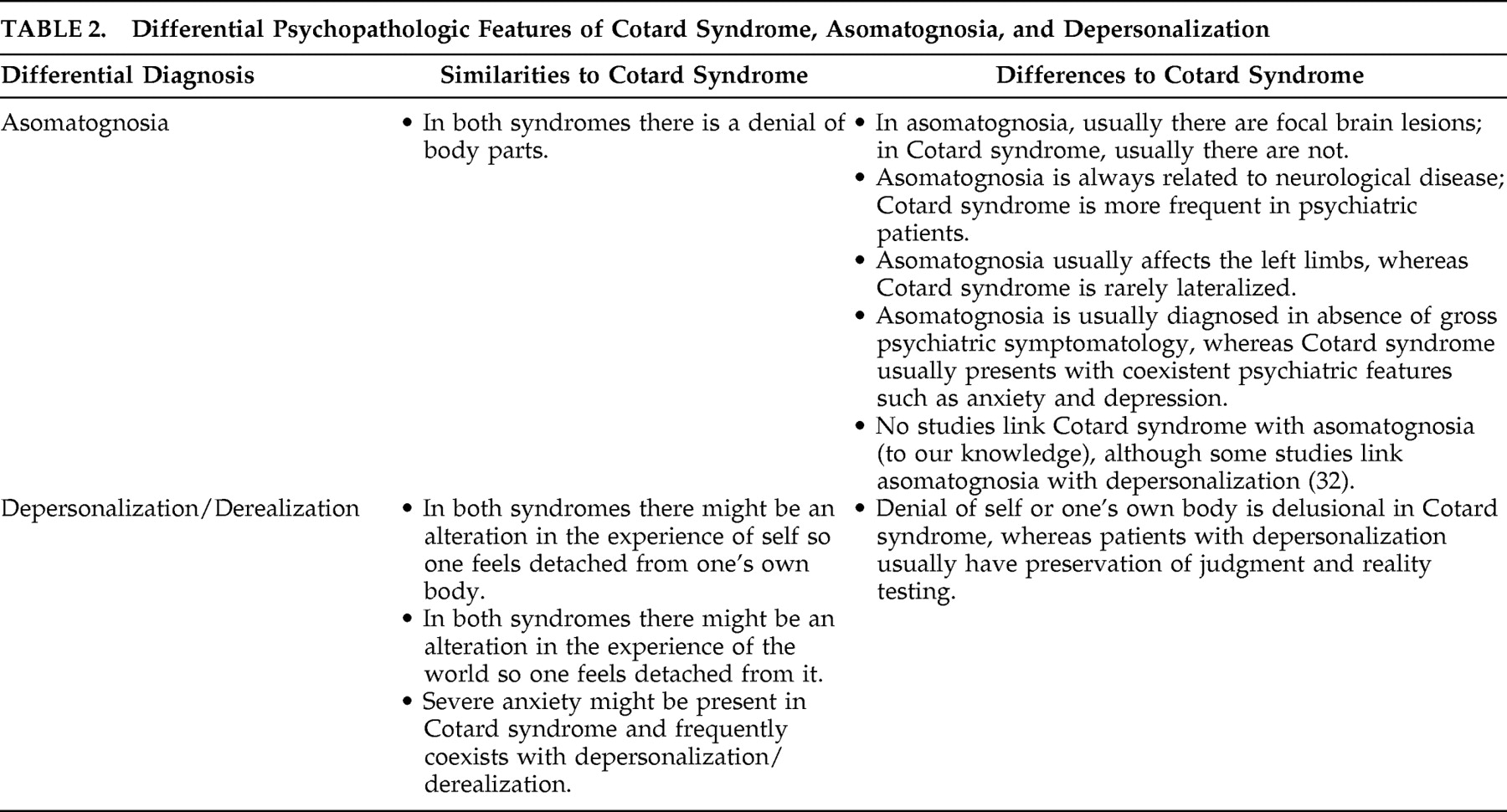
Clinicians must first distinguish Cotard syndrome from asomatognosia, or the neurological loss of awareness of parts of one’s body. All four patients have nihilistic delusions of body parts including the hands, heart, kidneys, bowels, eyes, and penis. Asomatognosia is the feeling that parts of the body are “missing,” are stolen, or have decayed.
24,
25 In patient 1, asomatognosia was probably an erroneous diagnosis because of bilateral characteristics of the symptoms, whereas asomatognosia is usually associated with right hemisphere lesions, and its clinical expression involves the contralateral body parts. Nevertheless, in patient 4 the nihilistic somatic delusion was lateralized, as the patient insisted that his left hand was dead. In this and similar patients, the somatophrenic subtype of asomatognosia may be indistinguishable from Cotard syndrome. The differential characteristics of Cotard syndrome and asomatognosia are summarized in
Table 2 .
Patient 4 had the additional unique syndrome of
koro, or the experience of penile retraction or disappearance.
26 To our knowledge, this feature has not been previously reported as a presentation for Cotard syndrome.
Koro is usually a culture-based belief associated with witchcraft or with panic episodes among groups of people, primarily in regions of Asia. In our patient,
koro is a further manifestation of the nihilistic delusions experienced as part of Cotard syndrome.
Explanation of Cotard syndrome includes the two-factor model of delusional beliefs.
6 First, patients with Cotard syndrome suffer from a highly unusual experience, possibly in the form of a loss of emotional experiences and a feeling of emptiness
27 ; patients with Cotard syndrome may have reduced or abnormal affective processing of perceptual experiences or the flow of information from sensory cortices to limbic-emotional areas.
28,
29 A complementary hypothesis is related to an imbalance of right-left anterior cingulate cortex, which can lead to lack or excess of feelings of familiarity for a perception.
30The second factor involves an impaired ability to evaluate candidates for belief in a proper way, often with a tendency to excessive internal attribution.
6,
27,
31 According to this thesis, the same unusual experience arising from abnormal affective processing could lead to the Capgras delusion, where relatives are believed to be impostors, if the patient employs an externalizing attributional style. Alternatively, if the patient uses an internalizing attributional style to interpret the same unusual emotional experience, he could develop Cotard syndrome. Although the contribution of attributional bias as a component of the “second factor” has received some empirical support,
5,
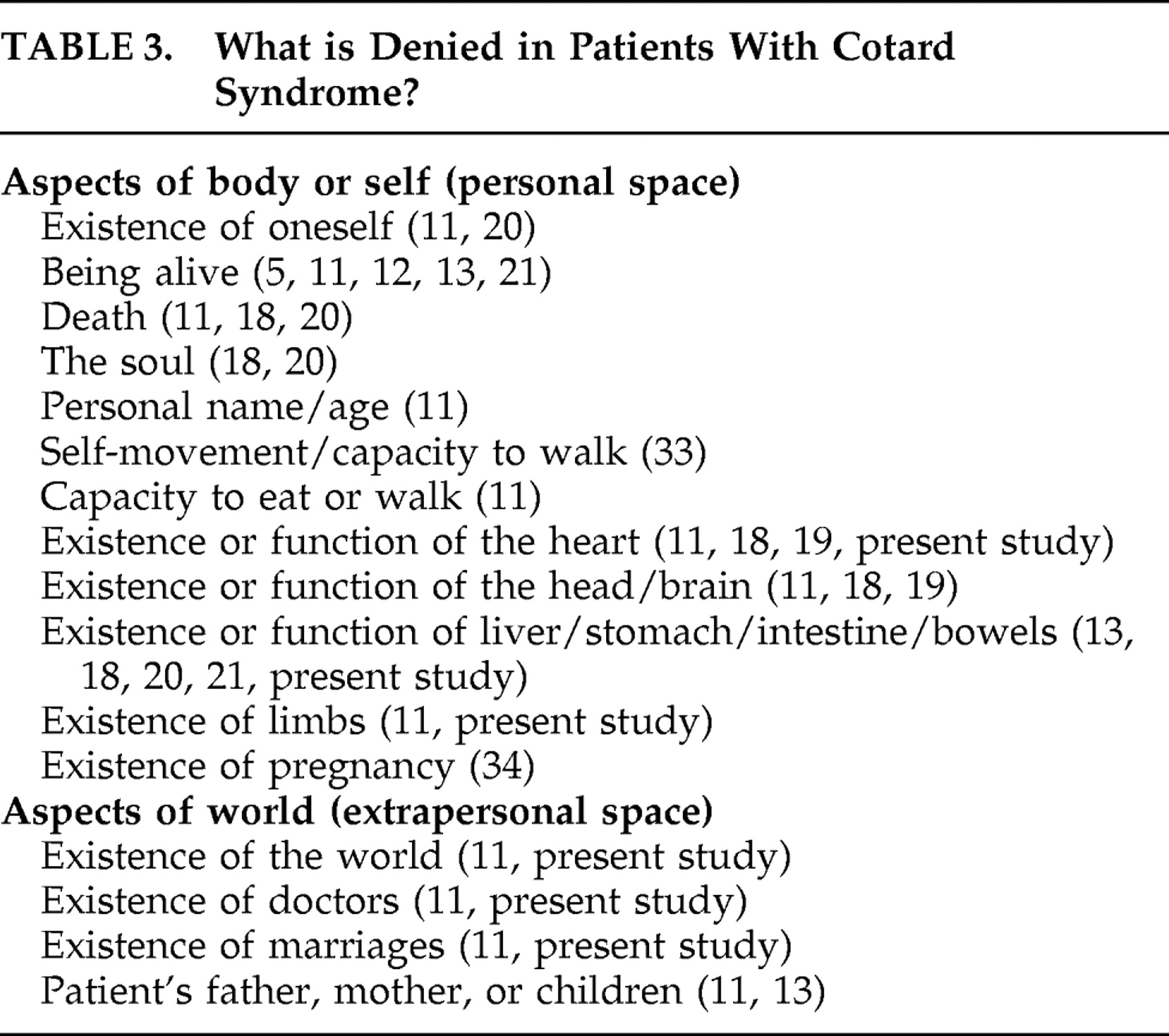
27 the role of self-misattribution in Cotard syndrome does not explain why some patients do not deny their whole self, body or life, but only parts of it, and why patients with Cotard syndrome frequently deny the existence of several objects or people in extrapersonal space
7,
11 (see
Table 3 ). The internalization misattribution bias hypothesis does not explain the coexistence of Capgras and Cotard syndromes in the same patient,
17 or the expression of self-nihilistic (internalized) and persecutory delusions (externalized) in the same person, as occurred in our patient 4 and according to classical reports.
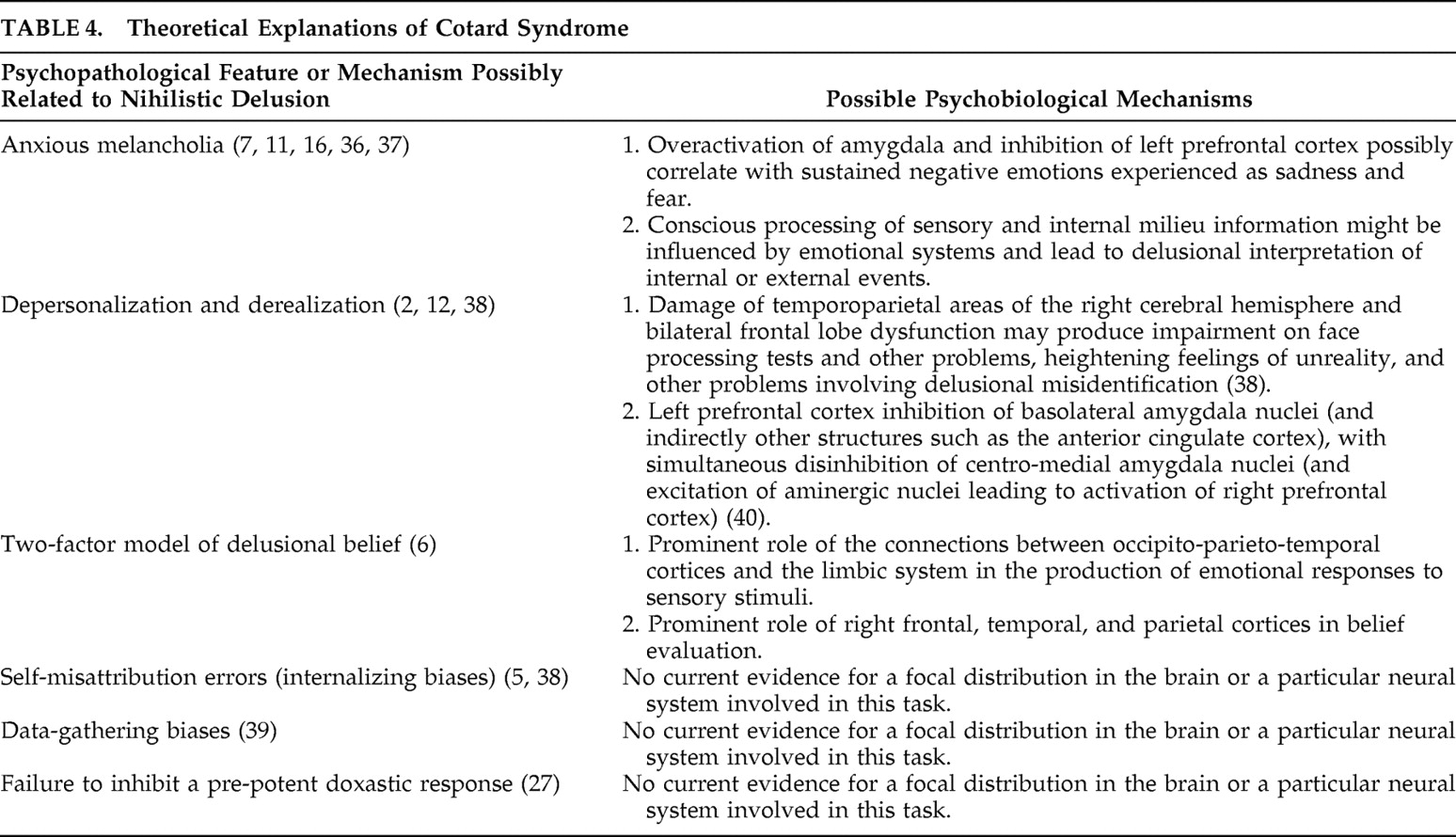
11The neuroanatomical view of Cotard and Capgras syndromes further challenges the misattribution hypothesis
4 (see
Table 4 ). According to this view, if disconnection occurs between the face recognition cortex and the limbic system, Capgras syndrome would be the clinical expression, but if all sensory areas are disconnected from the limbic system, Cotard syndrome might appear. According to this thesis, the right temporoparietal cortex
35 is the site of disconnection, whereas the right frontal lobe might have a significant role in belief evaluation.
6 Furthermore, it is possible to separate the features of depersonalization (a self-centered experience) from those of derealization (an experience referred to the world), by means of lesion analysis.
32 A patient with visual hypoemotionality secondary to bilateral occipitotemporal lesions could resemble a case of “isolated” derealization, whereas a patient with left asomatognosia due to a right frontal-parietal lesion could resemble a patient with depersonalization. Also, these facts provide theoretical background to the distinction between self-negation and external negation in patients with Cotard syndrome.
In our sample, 150 schizophrenia patients were included, but none of them showed clear nihilistic delusions. Cotard syndrome is well described in schizophrenia,
15,
17,
18,
21 but this feature might be infrequent (all previous reports of Cotard syndrome and schizophrenia are single cases or small case series). Regarding the possible neurobiological links between both conditions, and departing from the two-factor model,
6 nihilistic delusions could arise from highly abnormal experiences related to affective flattening or abnormal affective processing,
27 features often related to schizophrenia, and to the amygdala and cingulate cortex dysfunction,
40 as suggested by a case of self-mutilation and abnormal processing of pain in a patient with Cotard syndrome and schizophrenia.
21 As we know, the cingulate cortex is related to the affective component of pain.
40 Schizophrenia patients show prefrontal cortex dysfunction, which might be related to the deficit in critical evaluation systems predicted by the two-factor model.
Finally, psychotic depression or “anxious melancholia” (major depression with melancholic and anxious symptoms) plays an important role in many patients with Cotard syndrome. In one study, 89% of the classical cases presented with depression and 65% showed marked anxiety.
7 Psychotic or anxious depression could impact on the two factor model: pathological sadness and other phenomenological features of depression could account at least partly for the perceptual experience that constitutes the first factor, while they also provide an emotional bias for reasoning and belief evaluation. This does not, however, explain why the vast majority of patients with major depression, even psychotic or anxious depression, never develop Cotard syndrome. Moreover, a small but still considerable percentage of the Cotard patients do not show clear features of depression. For instance, an exploratory analysis
7 of 100 patients has found three subgroups of patients with Cotard syndrome: the first group had clear features of psychotic depression; the second was a more pure form, possibly related to delusional disorders; and the third group was mixed, with some anxiety, depression, and auditory hallucinations. Depression is neither necessary nor sufficient to explain Cotard syndrome, but it sets the most common clinical ground for the apparition of nihilistic delusions.
In conclusion, these four patients illustrate the frequency of Cotard syndrome in a large neuropsychiatric inpatient population and suggest a perceptual-emotional dissociation and a tendency to excessive self-attribution in the deliré des negations. Treatment strategies remain to be elucidated; however, all four patients described here had symptom resolution after treatment of their underlying neurological and psychiatric disturbances. Future research in monothematic syndromes like Cotard syndrome can elucidate how perceptions and their associated feelings interrelate in neuropsychiatric disorders.