P atients who fail to respond to conventional treatment for depression are classified as having treatment-resistant depression.
1 Lengthy trials of treatment with conventional medications may contribute to morbidity in patients with treatment-resistant depression, since it may take weeks or months to assess the effectiveness of each medication. Electroconvulsive therapy (ECT) is effective in only 80%–90% of unselected patients with depression and only 50% of patients with treatment-resistant depression.
1 Ketamine, a potent selective
N -methyl-
d -aspartate (NMDA) receptor antagonist,
2 has emerged as a promising agent in the treatment of patients with depression.
3 –
5 The treatment schedule used for ketamine, as well as populations of patients with depression, has varied from study to study. In one study,
4 ketamine (0.1 to 0.2 mg/kg/hour) was used in a continuous infusion for 5 days in two cases. Another study
3 of patients (n=7) with major depression compared treatments with ketamine (0.5 mg/kg) with saline, separated by a week, and found improvement for 72 hours after the ketamine infusion with a return to baseline levels of depression after 1 to 2 weeks. Similarly, a small randomized trial
5 in patients with treatment-resistant depression (n=18), in which ketamine (0.5 mg/kg) was used in a one-time dose followed by another dose 1 week later, found rapid improvement of depression with a 71% response rate and 29% remission rate lasting up to 1 week. However, the response rate dropped by one-half after a week.
In our pilot feasibility project, we assessed the effects of multiple ketamine treatments over a 12-day period on depression symptoms in two patients with treatment-resistant depression.
METHODS
We selected two adult patients with major depressive disorder without psychotic features confirmed by the Structured Clinical Interview for DSM-IV Disorders (SCID). These otherwise healthy participants had received multiple ECT treatments and had persistent symptoms of depression (Hamilton Depression Rating Scale [HAM-D] score ≥18). Adequacy of prior antidepressant treatment was determined using the Antidepressant Treatment History Form. Participants were hospitalized for a 12-day study period and were randomized to receive one of two infusion regimens: patient A: six 0.5 mg/kg infusions of ketamine (on days 1, 3, 5, 7, 9 and 11); patient B: two 0.5 mg/kg ketamine infusions (on days 1 and 7) and four saline infusions (on days 3, 5, 9, and 11).
Since the infusion dose in overweight subjects may cause perceptual distortions—because the dose is based on actual body weight—the Metropolitan Life Insurance tables were used to determine ideal body weight based on sex, age, height and body frame (http://www.coping.org/weightmgt/strategies/Weight%20Charts.doc).
On infusion days, physical and psychological assessments were conducted prior to infusion, every 15 minutes during the 40-minute infusion, and every hour for 3 hours postinfusion. Psychological assessments were conducted once on the off-infusion days. Perception and cognition were evaluated using the Clinician-Administered Dissociative States Scale, the Brief Psychiatric Rating Scale, and the Mini-Mental State Examination (MMSE). Laboratory tests were done at screening and at the end of the study including CBC, basic metabolic profile, liver function, and ECG. Depression symptoms were evaluated using the HAM-D and the Beck Depression Inventory (BDI). Posthospitalization evaluation of depression symptoms was conducted using the BDI by phone five times a week during the first 2 weeks posthospitalization and once a week thereafter. The study was approved by the SMDC Institutional Review Board.
Participants
Patient A is a 50-year-old man weighing 159.1 kg (ideal body weight=80 kg) with a history of depression since age 17. From 2003 to 2006 he had four hospitalizations for recurrent depression and, at times, concomitant suicidal ideation. Medical history included diagnoses of obesity and sleep apnea treated with continuous positive airway pressure. Over the 5 years prior to the study, he had 11 medication trials, each with a duration of at least 6 weeks. In 2005, he had seven right unilateral electroconvulsive treatments and reported no mood change. He refused any further ECT treatments. In general, the patient complained of a low mood, mood swings (from mild to significantly depressed), crying spells, social withdrawal, anxiety, difficulties with concentration, irritability, poor judgment and impulsiveness. He had no suicidal ideation at the time of the study. Screening with the SCID revealed no bipolar symptoms and no active chemical dependency or chemical abuse issues. Anxiety was not a predominant symptom.
Patient B is a 45-year-old man weighing 101.8 kg (ideal body weight=77.6 kg) with a history of depressive symptoms and treatment since the age of 10. He had been diagnosed with major depressive disorder, resistant type. He also had a history of alcohol abuse. Medical history included diagnoses of hypertension, and he had been treated with nine medications along with ECT. Between 2003 and 2007, this patient received a total of 105 ECT treatments (10 right unilateral treatments, 40 bilateral treatments, 23 modified bilateral treatments, 18 right unilateral/ultrabrief treatments, and 14 modified bilateral/ultrabrief treatments) and maintenance therapy. ECT produced both short-term and long-term memory loss without full recovery from the depression. In our study, the patient’s MMSE score was 30 at baseline, and no further cognitive testing was pursued. The subject received implantation of a vagal nerve stimulator in 2005.
RESULTS
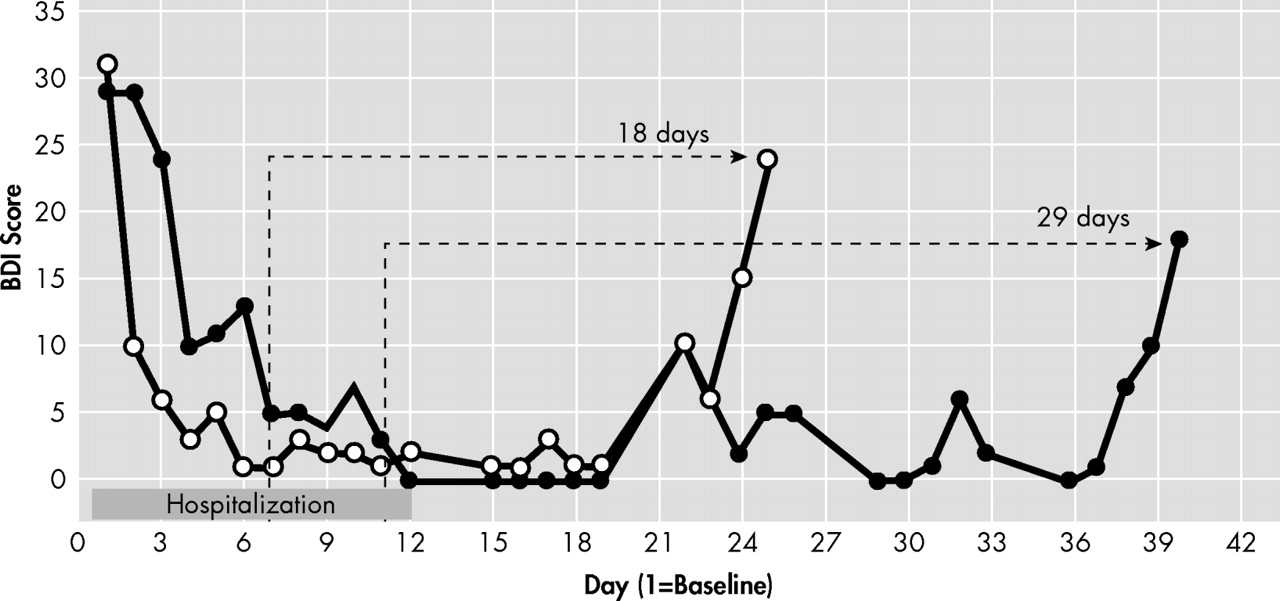
Both participants had marked changes in depression symptoms in response to ketamine treatment (
Figure 1 ) as measured by the BDI scores. Return of depression symptoms (BDI score >18) was observed on day 29 after the last ketamine treatment for patient A and on day 18 after the last ketamine treatment for patient B.
We observed no cognitive impairment, difficulties with memory, or problems with concentration associated with ketamine infusions. There was no respiratory suppression, and using ideal body weight for dose calculation prevented sedation. During infusions, 10 mm Hg elevation in both systolic and diastolic blood pressure was observed; the blood pressure returned to preinfusion values within 3 hours postinfusion. No hypoxia or problems with heart rate were noted. The ketamine infusions were not associated with short-term memory loss or confusion, which can be significant problems with ECT. The subjects exhibited a mild “intoxicating” effect during the ketamine infusions, with talkativeness and decreased inhibition. However, these symptoms did not persist beyond 2 hours after the cessation of the infusion.
Actual body weight was used for the calculation of ketamine dose for the initial infusion for patient A. However, the use of this dose was associated with visual misperceptions. All subsequent doses were calculated on the basis of ideal body weight. With the ideal body weight-based dose, patient A experienced intoxication without sedation and had no further abnormalities of perception.
The use of the lower dose did produce the desired antidepressant effect. The patients reported more immediate recovery with the ketamine treatment than they had experienced with ECT. These patients reported that the ketamine produced a marked change in mood that they did not experience with ECT treatments.
DISCUSSION
Compared with previous studies,
3,
5 in which a single dose of ketamine was used, our results suggest that there may be an extended time of recovery in response to multiple infusions of ketamine over time. The every-other-day dosing schedule used in this study was selected to assess the effects of repeated ketamine use on depression symptoms including mood change, cognitive function, sedation, psychosis and any potential physical side effects. The choice of the 2-week time period was based on the time needed to activate the cellular structure to produce adaptive changes seen with other treatments such as ECT.
6Ketamine may have several potential advantages over ECT for patients with treatment-resistant depression. One important advantage may be broader access. In our experience, ECT is not available in most areas and a referral must be made to a larger medical center for ECT treatment. In contrast, wherever psychiatric caregivers have the capacity to monitor an infusion process closely, ketamine treatment could be given. Ketamine infusions could also be safely administered on a psychiatric unit with proper monitoring and supervision. Use of ideal body weight for dose calculation provided safety without sedation or abnormalities of perception, yet assured an antidepressant effect.
As far as addictive potential, neither of the participants had any withdrawal symptoms nor was there any noted craving for the ketamine posttreatment. Since the infusions are in a highly controlled environment, attending psychiatrists can monitor both the treatment and any adverse effects of treatment, such as potential dependency. Thus, ketamine can be administered and managed on an inpatient psychiatric unit. Appropriate supervision during the infusion is necessary. Multiple treatments of ketamine may have a prolonged benefit for treatment-resistant depression patients. Further investigation of treatment strategies for best outcome is necessary to provide better care for patients with treatment-resistant depression.