Major depression is a common diagnosis in older individuals, with an estimated prevalence of 3%–4%,
1 and it is associated with a number of adverse health outcomes.
2–4 Rates of depression increase in individuals with cognitive disorders such as Alzheimer's disease (AD), where the prevalence is estimated to be as high as 26%, according to one recent study.
5Previous research looking at the results of antidepressant treatment in elderly patients suggests that it is effective in treating depression, both in patients with
6 and without
7 dementia, but that there is less evidence for patients with dementia. According to a recent review of antidepressants by Montgomery and colleagues,
7 published evidence indicates that SSRIs are likely the first-line treatment for depression in elderly patients without dementia, similar in effectiveness to older, tricyclic medications, but having a superior side-effect profile. Evidence on use in depressed patients with dementia, versus placebo, is less compelling but certainly exists for certain SSRIs, including citalopram and sertraline.
8,9The goal of this pilot study was to compare antidepressant treatment with sertraline in patients with and without dementia to assess whether treatment response varied with underlying diagnosis, using standard depression rating scales for patients both with and without dementia. We selected sertraline as the study medication because it had been previously used in clinical trials involving older persons.
9 We hypothesized, based on studies done to-date, that both treatment groups would respond, but that response would be greater in the non-dementia subgroup.
METHOD
This study received approval from the St. Michael's Hospital Research Ethics Board. Participants were recruited from several long-term care homes and the Outpatient Psychogeriatric Clinic at a large university teaching hospital. To be included in the study, participants had to have a Mini-Mental State Exam (MMSE) score above 16, meet DSM–IV criteria for major depressive episode, be willing and able to take antidepressant treatment, be above age 65, and speak English fluently. Participants were excluded if they were actively abusing substances, suffered from a mental illness other than major depression, had active CNS disease, or had unstable systemic medical disease. The diagnosis of AD was made in accordance with NINCDS–ADRDA criteria, whereas mixed dementia was diagnosed if AD was present with documented cerebrovascular disease. The diagnosis of major depression was made after clinical evaluation and according to established criteria.
A trained assistant interviewed participants and obtained consent according to ethical guidelines, took a detailed medical history, assessed global cognition (MMSE),
10 medical comorbidity (Cumulative Illness Rating Scale [CIRS]),
11 depressive symptoms (Hamilton Rating Scale for Depression [Ham–D],
12 Cornell Rating Scale for Depression in Dementia (CDD–D),
13 and quality of life (Dementia Quality of Life Scale [DQoL]).
14 After being screened for contraindications, all participants were started on an antidepressant treatment (sertraline), which was titrated from a starting dose of 25 mg to a minimum therapeutic dose of 50 mg and maximum dose of 200 mg over a period of 4 weeks. Titration would occur at a rate of 25 mg–50 mg every 4 days, as tolerated. After titration, participants remained on their target dose for a 12-week period and were seen on a biweekly basis after titration to monitor for drug tolerability. Depressive symptoms were measured monthly up to 16 weeks. Baseline cognitive (MMSE) and Quality of Life (DQoL) measures were also repeated at Week 16.
To compare group differences between the dementia (D) and non-dementia (ND) groups over time, we used a mixed-model approach.
15 Baseline characteristics were compared across the two groups with the independent-sample
t-test for continuous variables (e.g., age) and Fisher's exact test for categorical variables (e.g., gender). Statistical analyses were performed with SAS Version 9.2 (SAS Institute, Inc., Cary, NC). Significance was set at a probability level of 0.05, two-sided. Treatment response was defined primarily as change in score from baseline and, secondarily, as remission (CDD–D score <8 or a Ham–D score <7).
RESULTS
A group of 26 patients consented to participate in the study. Of these, 9 subsequently were later excluded for the following reasons: 1 patient passed away; 1 left the country; 4 were lost to follow-up; 2 decided to withdraw; and 2 no longer met the inclusion criteria when reassessed; 8 subjects met criteria for dementia, and 9 did not have dementia. Within the dementia subset, only one patient met criteria for mixed dementia; the remainder had probable AD. All patients remained on their target dose for a 12-week period.
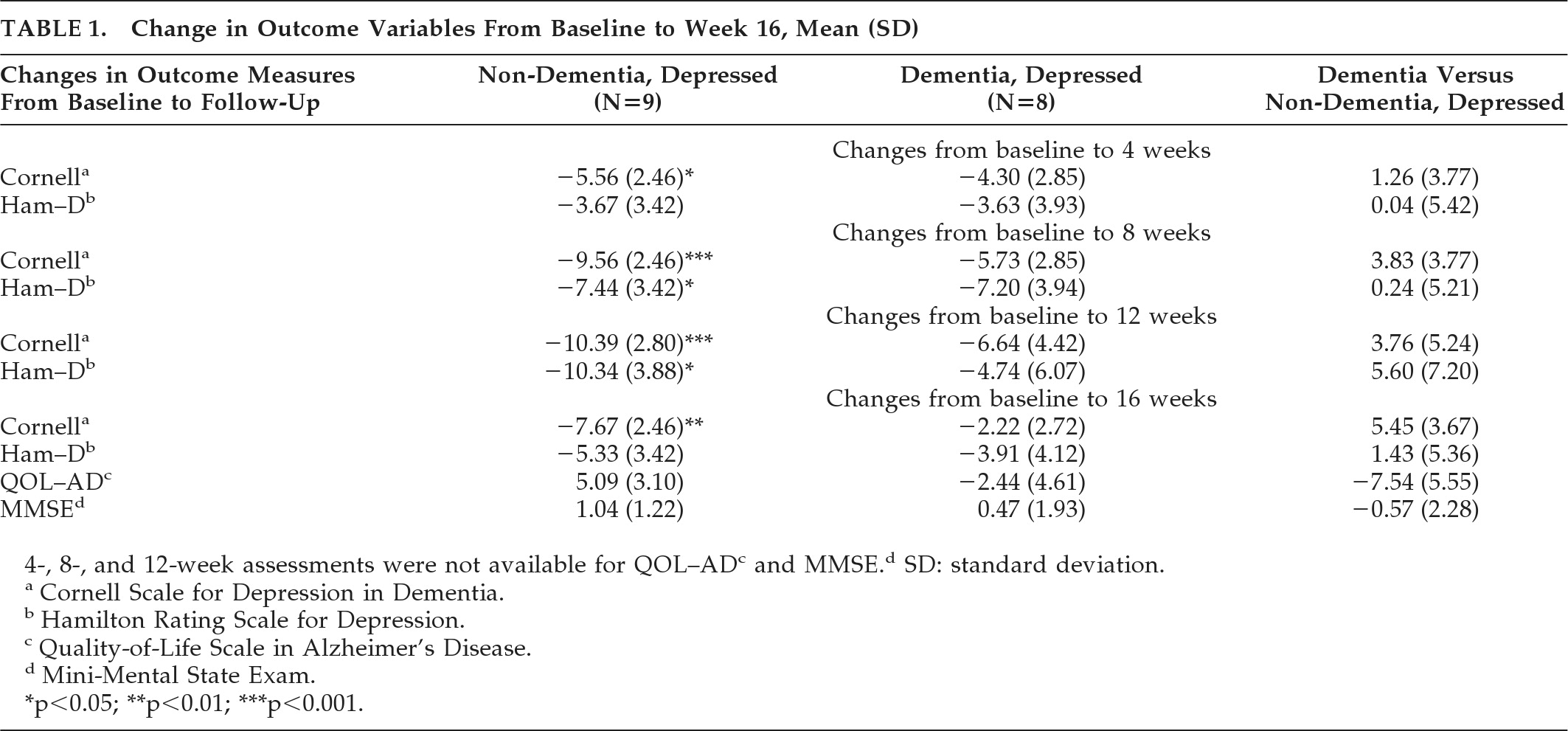
There were no significant differences between the two subgroups on any of the baseline data, including age (80.1 in ND versus 80.6 in D), gender (33.3% male in ND versus 50% male in D), baseline depression inventories (CDD–D score: 18.56 in ND versus 13.71 in D; Ham–D: 18.22 in ND, versus 17.29 in D), Quality of Life (ND: 28.56 versus D: 25.6) or Mini-Mental State Exam score (ND: 25.22 versus D: 18.3). Comparing treatment response in the dementia versus non-dementia subgroups, unfortunately, there were no significant differences detected between the two subgroups over time (
Table 1). However, the treatment response in the non-dementia subgroup reached statistical significance, whereas it did not in the dementia subgroup. This change was first noted in Week 4 (p<0.05), increased in Weeks 8 through 12 (p<0.001), and then decreased at Week 16 (p<0.01). The change was noted only on the CDD–D, and not on the Ham–D. The only significant difference in the Ham–D score was noted at Weeks 8 and 12 in the non-dementia subgroup (p<0.05).
In both subgroups, it was noted that depression scores improved in the initial 8 to 12 weeks of treatment, followed by a worsening of symptoms at Week 16. There were no significant differences detected within or between the subgroups with respect to other baseline variables, including QoL and MMSE. On the basis of the CDD–D score, remitters (N=7) were compared with nonremitters (N=8). No significant differences were noted between the two subgroups in terms of age, medication dose, or diagnosis, although remitters tended to be younger (83.3 years versus 85 years), have lower baseline CDD–D scores (14.8 versus 17.9), and be on a lower dose of sertraline (mean dose: 78.6 mg versus 111.1 mg) as compared with nonremitters. The range of sertraline used in the non-dementia subgroup was 50 mg–150 mg, and in the dementia subgroup was 50 mg–200 mg. In terms of adverse events, two patients (both non-dementia) developed dizziness, and one patient (non-dementia) developed headache secondary to the medication.
DISCUSSION
The aim of this study was to compare antidepressant treatment response in patients with and without dementia. As compared with baseline, scores on both depression inventories (the Ham–D and the CDD–D) improved over the 16-week course, although only the scores on the CDD–D approached statistical significance, in the non-dementia subgroup. These findings suggest that, consistent with the literature, depressed patients with dementia may improve on antidepressant treatment,
8,9 although treatment response may be less robust
6 than in depressed patients without dementia. One possible explanation for this is that depression associated with dementia may be more biologically-based.
13 It is interesting to note that antidepressant treatment response peaked at Week 12, then declined at Week 16. One potential reason for this is that patients were aware the clinical trial was finishing and thus would no longer be monitored as closely, possibly leading to an exacerbation of depression.
All depressed patients who started sertraline tolerated it well and felt subjectively better. Many could not tolerate levels of medication higher than 50 mg and may have benefited more were they able to. No one became suicidal after taking the medication, and it was well tolerated, with minimal side effects. Although this was a pilot study designed to generate data for future studies, there are some important findings. The study does suggest that although depressed persons with dementia may derive benefit from antidepressant treatment, depressed patients without dementia may derive significantly more benefit. Also, the CDD–D may be more sensitive at picking up differences in antidepressant treatment response between dementia and non-dementia depressed persons than the Ham–D. Furthermore, baseline medication dose or depression severity has little impact on treatment response; finally, drug studies in this population need to be sufficiently long (preferably over 16 weeks) to ensure that initial antidepressant treatment responses are sustained. Future studies should be designed with these modifications in mind.
Acknowledgments
This work was supported by the Drummond Foundation of Canada.