The introduction and evaluation of second-generation antipsychotics have dominated discussions about the psychopharmacology of schizophrenia since clozapine was first made commercially available in 1989 and since the subsequent marketing of risperidone (1993), olanzapine (1996), quetiapine (1997), ziprasidone (2001), and aripiprazole (2002).
Initially these medications were hailed as long overdue and dramatic improvements over their predecessors, although more recently doubts have been raised about their advantages in regard to side effects, effectiveness, and cost (
1,
2 ). This study examined changes in prescribing patterns for schizophrenia in a very large national health care system. It therefore complements previous efforts that examined the effect in the Department of Veterans Affairs (VA) of the introduction of ziprasidone for antipsychotic prescription (
3 ) and an earlier survey of the effect of the introduction of the class of second-generation antipsychotics in a Medicaid-funded treatment program (
4 ).
The availability of VA prescription data for patients throughout the United States provided a unique opportunity to examine antipsychotic prescribing trends for schizophrenia.
Methods
As an ongoing summary of the pharmacotherapy of VA patients with schizophrenia, annual reports are compiled on antipsychotic use and formed the basis of our study (
5 ). For each federal fiscal year (FY; October 1 to September 30) from 1999 through 2006, all patients diagnosed as having schizophrenia were identified through national VA administrative databases. Patients met the operational definition of schizophrenia if they had at least two outpatient encounters in a specialty mental health outpatient clinic and a primary or secondary diagnosis of schizophrenia (
ICD-9 codes 295.00–295.99), as recorded in the outpatient encounter file documenting information on every outpatient visit throughout the VA or in an inpatient discharge abstract. The pharmacy records of all patients fulfilling this operational definition of schizophrenia were also obtained from administrative databases, and prescriptions for antipsychotic medications filled in the VA were extracted. The last prescription for an antipsychotic medication filled in each FY was identified as the index prescription, and all prescriptions recorded for the seven days before the index prescription were included. The second-generation antipsychotics examined for this report included olanzapine, risperidone, quetiapine, clozapine, aripiprazole, and ziprasidone, and the first-generation antipsychotics, considered as a group, included chlorpromazine, fluphenazine, haloperidol, loxapine, mesoridazine, molindone, perphenazine, prochlorperazine, thioridazine, thiothixene, and trifluoperazine.
Polypharmacy applied to less than 10% of patients. Those who were prescribed both a first-generation antipsychotic and a second-generation antipsychotic were categorized according to the second-generation antipsychotic they were receiving. Therefore, patients in the first-generation antipsychotic category were receiving this class of medication as the sole antipsychotic prescribed, although they could have received more than one member of the class. Long-acting medications were not included in this analysis and are used by about 12% of VA patients with a diagnosis of schizophrenia (
6 ).
Results
In FY 2006, 88,507 patients with a diagnosis of schizophrenia were identified. This represented a decrease of 14.1% from the number of patients treated in FY 1999. Of these patients 78,849 (89.1%) were prescribed an antipsychotic medication, and 9,658 (10.9%) were prescribed more than one antipsychotic.
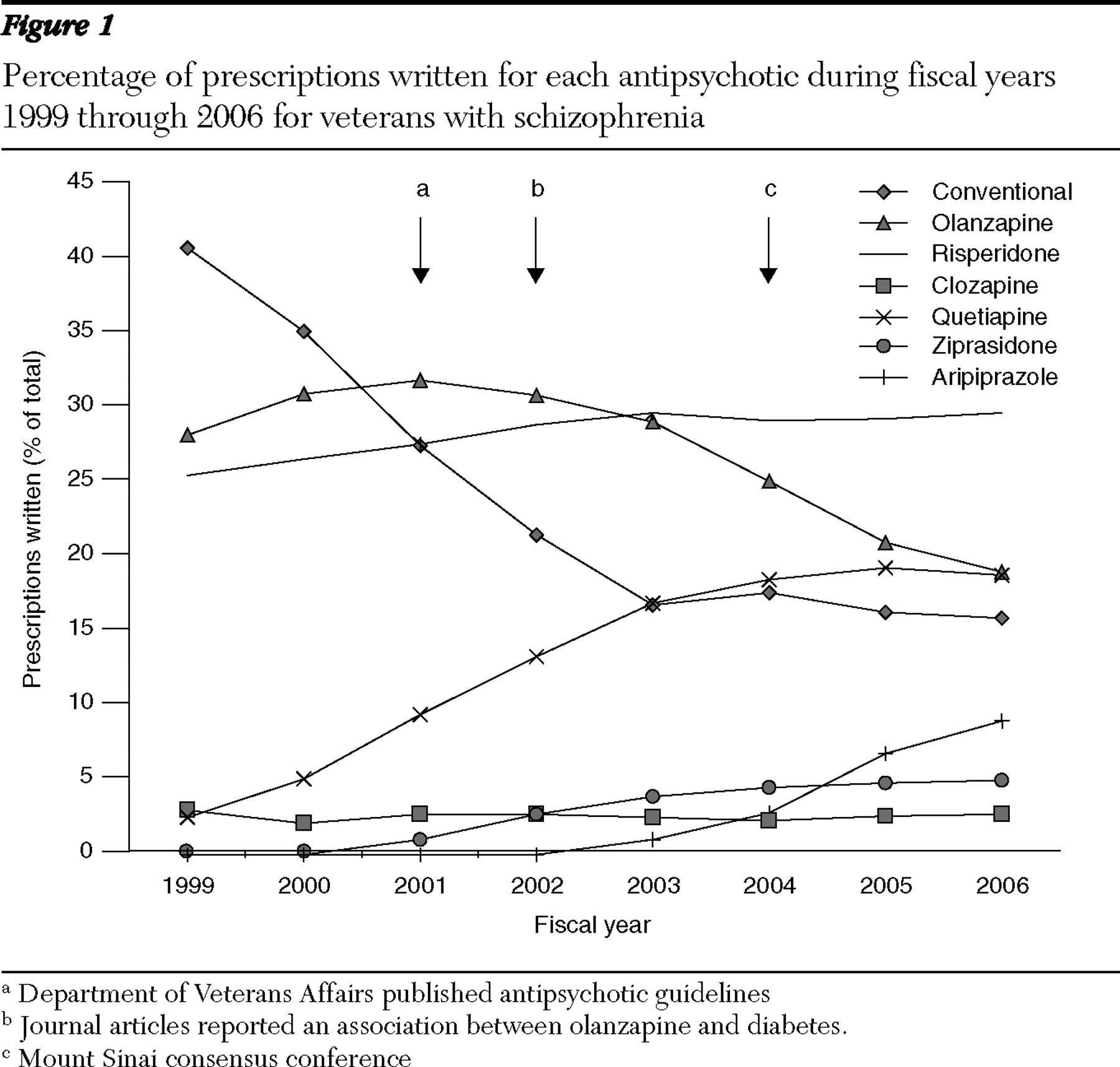
The percentages of patients with schizophrenia prescribed each of the medications in each of the years are presented in
Figure 1 . The percentage of patients prescribed first-generation antipsychotics decreased from 40.8% (FY 1999) to 16.8% (FY 2003), an annual average decrease of 6%, and remained nearly level over the next three years, ending at 15.9% for FY 2006.
Use of each of the second-generation antipsychotics consistently increased except olanzapine, which increased by 4.0% in the first two years but then decreased every subsequent year at an average rate of 2.6% per year. The proportion of patients prescribed olanzapine thus increased from 28.2% (FY 1999) to 31.9% (FY 2001) and then decreased in each subsequent year, to 18.8% in FY 2006. The proportion of patients using risperidone increased from 25.5% (FY 1999) to 29.7% (FY 2003) and was virtually constant thereafter. Patients prescribed quetiapine increased from 2.5% (FY 1999) to 18.5% (FY 2004), with little change in the past two years. Ziprasidone, first available in FY 2001, was initially used by 1.0% of patients and increased to 5.0% by FY 2006. Use of aripiprazole, first available in FY 2003 (1.0%), increased every year until FY 2006, when it reached 9.0%. Patients taking clozapine accounted for 2.1% to 3.0% of all prescriptions throughout the study period.
Discussion
Several patterns of use are apparent from these data. First, medications introduced since national data became available for VA prescriptions (for aripiprazole and ziprasidone) or just before that time (for quetiapine) show steady growth in proportionate share. Quetiapine and aripiprazole use proceeded on roughly parallel curves, whereas growth in use of ziprasidone was more modest, so that in FY 2006 prescription rates for aripiprazole were nearly three times that for ziprasidone despite having been introduced two years later than ziprasidone. The much shallower slope of the ziprasidone curve may be due to the extensive publicity at the time of its introduction about its association with QTc interval prolongation on electrocardiograms. Within the VA there was a specific recommendation that it not be used with patients who had an underlying risk for QTc interval prolongation (
7 ).
The two second-generation antipsychotics that were introduced earliest (clozapine and risperidone) had relatively little change in their rate of prescription over the years studied. After some initial variability, the percentage of patients using clozapine has been consistently low, and the percentage of patients receiving risperidone, released in 1993, grew slowly throughout the study period.
Perhaps the most striking patterns of change are that olanzapine and first-generation antipsychotics decreased proportionately, albeit for potentially different reasons. The growth in use of newer medications appears to have come at their expense.
The percentage of patients prescribed olanzapine over the study period grew modestly over the first two years, but after FY 2001 its use began to decline and dropped steadily over the next five years. There are two potential explanations for this observation. First, this decrease might be in response to published concerns about the risk of weight gain and diabetes. The earliest publication linking olanzapine and diabetes was published in 1998 (
8 ), and articles describing drug surveillance reports (
9 ) and the increased association of olanzapine and diabetes in a large VA sample (
10 ) both appeared in 2002. In 2004 the results of a consensus conference identified olanzapine, along with clozapine, as being particularly prone to inducing weight gain, diabetes, and worsening of lipid profiles (
11 ). A recent search of the Thomson Scientific's Web of Knowledge for articles on olanzapine and diabetes yielded 265 articles, and this issue has recently been taken up by the lay press (
12 ). The first widely available article addressing weight gain associated with olanzapine appeared in 1999 (
13 ).
A second explanation is the publication of a guideline for VA physicians (
14 ) that stated that "there is no consensus in the literature to support one [second-generation antipsychotic] being globally superior to another" and that the absence of patient-specific factors "at the present time . . . would lead to the preference of quetiapine and risperidone over olanzapine." It is important to note that although these guidelines neither establish a "fail first" policy for olanzapine nor recommend changing effective regimens, they also did not prevent individual medical centers from facilitating the use of risperidone or quetiapine.
The proportion of patients prescribed first-generation antipsychotics fell by FY 2003 to approximately one-third of FY 1999 levels and then remained flat. The flattening of the curve might be explained by the publication of a large VA-sponsored study in 2003 (
2 ) that showed no advantage of olanzapine over haloperidol. However, this study was not extensively publicized and thus is not likely to have had an observable effect on prescribing practice in the VA. There is no evidence in these data of a return to prescribing first-generation antipsychotics despite recently publicized studies that failed to demonstrate a marked superiority of the second-generation antipsychotics versus haloperidol (
2 ) and perphenazine (
1 ). More specifically, the percentage of patients receiving perphenazine in FY 2005, the year before the first published results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), was 1.8% and remained the same the next year.
Several more general observations can be made. First, it would appear from these data that large, evidence-based studies such as CATIE have not yet influenced practice as measured by antipsychotic prescription. This is consistent with recently published data from a review of new prescriptions in a large pharmacy database (
15 ). Second, the decrease in the proportion of first-generation antipsychotics appears consistent with one generation of medications being supplanted by another, in this case occurring in the context of extensive marketing and the legitimate wish of prescribers to reduce the risk of neurological side effects. Third, the timing of the decline in olanzapine appears consistent with increasing concerns about side effects and VA pharmacy policies.
There are several limitations to this study. First, these data address the prescription of these medications only for VA patients with a diagnosis of schizophrenia. Within the VA only approximately half of the patients receiving antipsychotic medications are diagnosed as having schizophrenia (data available from the second author). Second, although the yearly prescription rates are available, specific reasons why particular medications are chosen or avoided are not available from these data sets. Although the VA has produced recommendations for antipsychotic use, no nationally coordinated attempts were made to restrict availability of these medications. Third, although the VA is the largest integrated care delivery system in the United States, the comparability of these trends to psychiatric practice elsewhere is unknown.
In our view, analysis of antipsychotic prescribing patterns over an eight-year period suggests that concerns about side effects are the strongest driver of differences in medication use.
Acknowledgments and disclosures
This work was supported by the Department of Veterans Affairs Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center.
Dr. Rosenheck has received research support from Eli Lilly and Company, Janssen Pharmaceutica, Astra-Zeneca, and Wyeth. He also has been a consultant to GlaxoSmithKline, Bristol-Myers Squibb, and Janssen Pharmaceutica. He was retained as an expert witness on behalf of plaintiffs in their suit against Eli Lilly and Company. Dr. Sernyak has received honoraria from Pfizer.