Patients in treatment for depressive disorders are at much higher risk of suicide than the general population (
1,
2,
3,
4 ). Patients with depression have even higher rates of completed suicide after psychiatric hospitalization (
3,
5,
6,
7 ), and several studies indicate that patients with depression have higher rates of suicidality (ideation and attempts) after antidepressant starts and dosage changes (
8,
9,
10 ). Close monitoring during these periods may reduce suicide risk.
Clinical monitoring of the population being treated for depression in the Department of Veterans Affairs (VA) health system is important to investigate because suicide rates in this system are higher than the rates in the age- and gender-matched U.S. population, the patient population is large, and systemwide reforms are feasible (
1 ). The rate of completed suicide among VA patients receiving depression treatment between fiscal years (FYs) 1999 and 2004 was 114 per 100,000 person-years (per year of life observed) in the 60-week periods after antidepressant starts, dosage changes, or psychiatric hospitalization. Completed suicide rates in the first 12 weeks after these events were higher at 568 per 100,000 person-years after psychiatric hospitalization, 210 per 100,000 person-years after new antidepressant starts, 193 per 100,000 person-years after other antidepressant starts (in ongoing treatment), and 154 per 100,000 person-years after antidepressant dosage changes (
11 ).
Although close clinical monitoring has not been proven to reduce suicide, it is often suggested to reduce suicide risk. The U.S. Food and Drug Administration (FDA) has recommended close monitoring in the 12 weeks after antidepressant starts or dosage changes to reduce suicide risks during these periods (
12 ). Although the agency's recommendations have undergone multiple iterations and have not always specified a visit frequency, the most stringent recommendation was for seven visits in the 12-week period after antidepressant starts or dosage changes for youths, with a later recommendation that adults with major depression be observed similarly (
9,
10 ).
The visit frequency suggested by the FDA to reduce suicide risk is similar to that suggested by past treatment guidelines to increase treatment efficacy during important treatment periods, such as those after depression treatment initiation (
13,
14 ). However, depression treatment patterns have changed substantially in the past ten to 15 years. The number of patients receiving depression treatment has increased while the number of visits per patient has decreased (
15 ). In this changing context, many clinicians have viewed the FDA monitoring recommendations as being logistically difficult and prohibitively costly to implement. Several studies have reported decreased rates of new antidepressant prescriptions since the FDA warnings (
16,
17,
18 ), although other concurrent events may have contributed to this decline.
In a world of scarce health care resources, policy makers are increasingly considering the costs and benefits of proposed health care interventions. For example, studies have examined the cost-effectiveness of screening mammography in various age groups and at different intervals, child health screenings, and other preventive services (
19,
20,
21,
22,
23,
24 ). Solid research evidence regarding the effectiveness of increased monitoring in reducing suicide is not yet available. However, given governmental and other organizational recommendations to provide close monitoring during high-risk periods, it is important to assess the potential service implications and the costs of following these recommendations.
We examined the frequency with which high-risk treatment periods occur in a nationally representative sample of VA patients in depression treatment and the intensity of monitoring provided to patients during these periods. We then examined the extent of service changes that would be required and the incremental costs of providing monitoring consistent with the most stringent FDA recommendation for monitoring. We hypothesized that monitoring rates are less intensive than the most stringent FDA recommendation and that the costs of providing monitoring consistent with FDA recommendations would be substantial.
Methods
Study data came from the VA National Registry for Depression (NARDEP), which is a unique longitudinal data resource that includes data from multiple VA administrative data sources and provides detailed services and pharmacy data for more than 1,500,000 patients diagnosed as having depressive disorders in VA facilities from FY 1997 and afterward.
Patient population and observation days
We randomly selected 100,000 of the 887,859 patients in NARDEP who received either two depression diagnoses or a depression diagnosis and an antidepressant fill between April 1, 1999, and September 30, 2004. Depression diagnoses were identified by using the following ICD-9 codes: 293.83, 296.2x, 296.3x, 296.90, 296.99, 298.0, 300.4, 301.12, 309.0, 309.1, and 311. Patients were excluded if they received diagnoses of bipolar I disorder, schizophrenia, or schizoaffective disorder during this period.
Observation began on the date of patients' second depression diagnosis or their antidepressant fill and continued until their date of death in the National Death Index, one year after their last VA services use, or the study end date (September 30, 2004), whichever occurred first.
Treatment events
As in prior studies, we ascertained dates of discharge from psychiatric hospitalization and the dates of antidepressant fills during the observation period (
11 ). We also ascertained new antidepressant starts, defined as fills occurring after a "clean period" of six or more months without any antidepressant fills. Other antidepressant starts were fills occurring during ongoing treatment, either without a clean period or with a short clean period of less than six months (that is, antidepressant switches, initiation of antidepressant combination treatment, or new medications after a short gap in use). A significant change in antidepressant dosage was defined as a change of ≥50% in total dosage of a specified antidepressant between two consecutive fills occurring in a six-month period (
11 ).
Risk periods after treatment events
We classified patient observation days by whether they fell within sequential 12-week period after each type of treatment event. Days were classified as being within one to 84 days (12 weeks), 85 to 168 days (13–24 weeks), or ≥169 (>24 weeks) after the event.
Monitoring visits
We defined depression monitoring visits on the basis of criteria specified in the Healthcare Effectiveness Data and Information Set (HEDIS) optimal practitioner contact measure, as modified for VA settings (
25 ). All visits with mental health Current Procedural Terminology (CPT) codes were counted as depression monitoring visits. Visits with non-mental health CPT codes were also counted if they had an associated
ICD-9 depression diagnosis code of 290, 293–302, or 306–316. In sensitivity analyses, we explored the effect on study findings of using alternative variable definitions, counting all mental health and primary care visits as monitoring visits regardless of the accompanying
ICD-9 codes.
We calculated the mean number of monitoring visits completed per 12-week high-risk period after each type of treatment event by calculating the ratio of completed monitoring visits divided by the number of high-risk observation days after each type of treatment event and then by multiplying this ratio by 84 to give us the average number of visits in 12-week high-risk periods across patients and treatment events over the entire observation period.
We also determined the frequency and mean number of HEDIS qualifying visits after patients' first antidepressant event of interest and their first psychiatric hospitalization. Patients were excluded from this analysis if they did not have the treatment event of interest or if they had less than 84 observation days after this event.
Costs associated with increasing monitoring visits
We used VA Health Economics Resource Center (HERC) average cost data to estimate incremental costs that would be required to provide intensive monitoring during high-risk treatment periods. HERC average cost data were developed for use in health economics research and have been used in several prior studies (
26,
27,
28 ).
We determined the treatment costs associated with increasing to seven the number of visits for patients who had fewer than seven visits during high-risk treatment periods for suicide (such visits are referred to below as incremental visits). These analyses did not assume visit reductions among patients with more than seven visits during these periods.
Because health care organizations may increase the level of monitoring by adding less expensive visits or by adding visits that reflect the current practice mix, we first estimated treatment costs for an inexpensive incremental mix. In these analyses we assumed that for every three incremental visits, two would be telephone visits (CPT codes 99371, 99372, or 99373 at a cost of $30.32 per visit) and one would be a 15-minute office visit (CPT code 99213 at a cost of $121.67 per visit). This assumption is congruent with the National Committee on Quality Assurance quality monitor for optimal practitioner contact after new antidepressant starts (
25 ).
We then estimated the cost of increasing visits up to seven on the basis of the visit mix currently offered to VA patients in depression treatment. Eighty percent of depression monitoring visits in VA settings during FY 2004 were accounted for by the following CPT codes: 90801, 90804, 90805, 90806, 90807, 90853, 90862, 99212, 99213, and 99214. On the basis of this practice mix, the average cost of a monitoring visit during the 12-week period after an inpatient stay was $99.09 and the average cost of a monitoring visit after an antidepressant change was $115.43.
Other potential costs
Although HERC average cost data include the average overhead costs of visits (for example, hiring and training clinicians, office costs, and outreach to patients), these estimates do not include pharmacy costs. Antidepressant costs may increase if closer monitoring results in more medication adjustments and increased adherence. To estimate potential incremental pharmacy costs associated with closer monitoring, we multiplied the mean cost of antidepressants in the first 12-week high-risk periods during FY 2004 ($121.42) by the observed increases in antidepressant adherence (15%–65%) reported during collaborative care interventions for depression (
29,
30,
31 ).
Estimating overall costs
To determine the incremental costs to the VA health system of providing close monitoring during all high-risk treatment periods in FY 2004, we determined the incremental costs of bringing cohort patients who had their most recent VA visit within one year of FY 2004 up to seven visits during all high-risk periods in FY 2004. We then extrapolated these costs to the 807,891 VA patients who had received depression treatment during the study period and remained in active treatment within one year of FY 2004.
Data analyses
We completed descriptive statistics for the patient sample, using frequencies or means as appropriate. Other data analyses are described above.
Results
Patient characteristics
The characteristics of the patient cohort are outlined in
Table 1 . Patients had a mean age of 58.6 years and were predominantly male (92%).
Hospitalization, antidepressant starts, and dosage changes
Table 2 presents the percentages of cohort patients who had a psychiatric hospitalization, received antidepressant medication, had an antidepressant start, or had a dosage change during the observation period. A total of 82,677 patients (83%) had at least one antidepressant start, dosage change, or psychiatric hospitalization. On average, patients experienced .94 treatment events per patient-year of observation (95% confidence interval [CI]=.94–.95).
Monitoring intensity
On the basis of the HEDIS definition of monitoring visits, patients averaged 4.86 visits (CI=4.82–4.89) in the 12-week period after psychiatric hospitalization and 2.40 visits (CI=2.39–2.40) in the 12-week period after antidepressant treatment events. They completed an average of 2.18 visits (CI=2.16–2.19) after new antidepressant starts, 3.02 visits (CI=3.00–3.03) after other antidepressant starts, and 2.64 visits (CI=2.63–2.65) after significant dosage changes.
In sensitivity analyses, when all mental health and primary care visits were assumed to be monitoring visits, patients completed an average of 5.66 visits (CI=5.62–5.69) in the 12-week period after psychiatric hospitalization and 3.08 visits (CI=3.07–3.09) in the 12-week period after antidepressant treatment events.
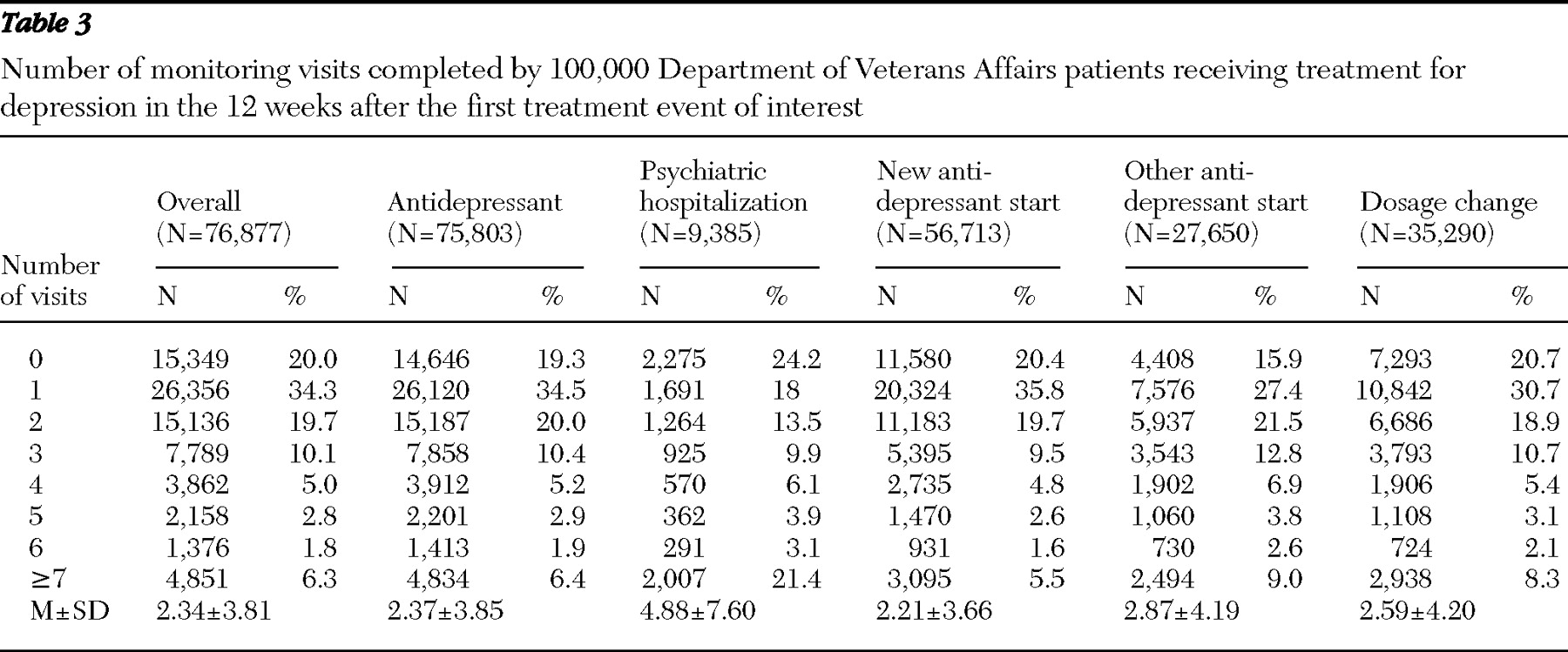
Table 3 presents the frequency and mean number of HEDIS qualifying visits after the first treatment events of interest among 76,877 patients with at least one of the specified events and ≥84 days of follow-up after this first event. Only 6.4% of patients who had an antidepressant treatment event completed seven or more visits in the 12-week period after this first event; approximately 21.4% of those with a psychiatric hospitalization completed seven or more visits in the 12-week period after this first hospitalization.
Estimated incremental costs of monitoring
Depending upon whether additional visits reflected an inexpensive mix or the observed mix of monitoring visits, increasing the number of visits to seven for patients with less intensive monitoring during high-risk periods would mean an additional cost of approximately $408–$537 for each high-risk period after an antidepressant treatment event or $313–$341 for each high-risk period after a psychiatric hospitalization.
For patients who received depression treatment and remained in VA care, approximately 3.5 additional visits would have been required to reach seven visits, at a cost of $303–$394 per patient-year.
Incremental antidepressant costs
Among the 25,754 patients who had their first high-risk period in 2004, mean antidepressant costs during the 12-week high-risk period were $121.42. If closer monitoring increased antidepressant costs by 15%–65% as a result of improved adherence, this would represent incremental costs of $18–$79 per high-risk treatment period.
Estimated overall costs
For all 807,891 patients receiving depression treatment since 1999 and remaining in active VA care in FY 2004, the health system would have had to expend an additional $183–$270 million in 2004 dollars to provide intensive monitoring during all high-risk periods during the year. Increased antidepressant adherence would potentially add another $.9–$3.8 million to this figure. To provide intensive monitoring only during the treatment periods after psychiatric hospitalization would have cost $15–$17 million in 2004 dollars.
The incremental costs of providing close monitoring during all high-risk periods for patients in depression treatment would have represented .7%–1.0% of the overall FY 2004 VA health care budget of $27 billion. Providing close monitoring only during treatment periods after psychiatric hospitalization would have represented approximately .06% of the overall health care budget (
32 ).
Discussion
Close monitoring is frequently suggested as a way to reduce suicide risk, and the FDA has specifically recommended close monitoring for this purpose after antidepressant starts and dosage changes. We present data on the potential service changes and costs of implementing intensive monitoring during high-risk periods for suicide in the nation's largest health care organization.
We found that patients receiving VA depression treatment had less than one high-risk treatment period during each year of follow-up. They completed an average of 2.4 monitoring visits in the 12 weeks after antidepressant starts and dosage changes and 4.9 visits in the 12 weeks after psychiatric hospitalization. Given high rates of completed suicide among the VA depression treatment population (
1,
11 ), closer monitoring might be needed.
The VA would need to substantially reorganize depression services to provide monitoring consistent with the most stringent FDA recommendations. To provide seven visits during these 12-week high-risk periods, the average number of visits would need to be almost tripled in the 12 weeks after antidepressant starts and increased by 40% in the 12 weeks after psychiatric hospital discharge.
This would cost an additional $408–$537 per high-risk period after antidepressant treatment events and $313–$341 per high-risk period after psychiatric hospitalization. On average, this would result in additional expenditures of $303–$394 per patient-year of treatment. The lower range of estimated costs would result if two telephone-based visits were included in each of three incremental visits. Telephone visits are less expensive, and there is substantial research indicating the effectiveness of telephone-based care in improving outcomes for patients newly starting antidepressants (
29,
31,
32,
33 ). Thus telephone visits may also play a role in efforts to reduce suicide.
With any visit mix, the incremental aggregate costs of providing intensive monitoring are substantial. Health systems with limited resources might need to provide intensive monitoring first during the highest-risk treatment period—that is, after psychiatric hospitalization. The costs of providing intensive monitoring at this critical juncture are more modest, and suicide rates during the 12 weeks after hospitalization are very high, at 568 per 100,000 person-years.
Given the substantial aggregate costs of implementing intensive monitoring during high-risk periods for suicide, there is also an urgent need for further research regarding the effectiveness of close monitoring in reducing suicide. A recent review of suicide prevention studies concluded that only physician education and restricting access to lethal means have reasonable evidence for efficacy in preventing suicide (
34 ). Health care organizations will likely require solid evidence for the effectiveness of intensive monitoring in reducing suicide risk before making the large shifts in depression services required for widespread implementation. Future research must also address the frequency, nature, duration, and content of monitoring visits that are most effective in reducing risks.
We conducted this study with data from VA patients in depression treatment; care must be taken in generalizing study findings. The VA population may be at higher risk of suicide than many treatment populations, although suicide rates for men receiving VA depression treatment are close to those of men receiving depression treatment in managed care settings (
2 ). Because of high rates of comorbidity, VA patients may also receive more antidepressant changes and be hospitalized more often than other treatment populations, resulting in more high-risk periods. VA patients may also receive more or less frequent monitoring visits than patients in other settings. However, the percentage of VA patients completing seven or more mental health-focused visits in the 12 weeks after antidepressant treatment events was quite similar to the percentage of managed care patients completing seven or more such visits after new antidepressant starts (
35 ).
Hiring new staff would likely be necessary to increase overall visit frequency during high-risk periods. Although HERC average cost data include the average overhead costs involved in hiring and training new staff, a significant and sudden expansion might result in a temporary cost increase that is not fully captured. HERC cost data also include the average costs of outreach needed to bring patients in for visits, but outreach efforts needed to bring patients in for intensive monitoring may differ from these average outreach costs (
36 ).
In this study we focused on reducing the suicide risk of patients in depression treatment. Other populations of patients, such as those with bipolar disorder or schizophrenia, may also benefit from closer monitoring during high-risk periods.
Finally, health systems may configure incremental visits in a number of ways to meet FDA monitoring recommendations. In this study we presented the least and most expensive options that we thought health systems might consider. However, the optimal visit mix for reducing suicide risk is not known. Potentially, only visits with psychiatrists or skilled psychotherapists might prove effective in reducing suicide. The costs of providing intensive monitoring composed only of these expensive visits would fall outside of the range presented. Alternatively, new technologies such as sending text messages to patients (via phone or computer) or other automated monitoring systems may be feasible and result in costs lower than those presented here.
Conclusions
Despite the study limitations, data from this national cohort of VA patients in depression treatment provide important information regarding the frequency of hospitalization, antidepressant starts, and dosage changes in clinical settings and the intensity of clinical monitoring after these events, which allows data-driven estimates of the potential service changes and costs of providing intensive monitoring during high-risk periods for populations receiving depression treatment. These estimates can serve as benchmarks for assessing tradeoffs, as we learn more about the clinical effectiveness of intensive monitoring in reducing suicide risk.
Acknowledgments and disclosures
This research was supported by grants IIR 04-211-1 and MRP 03-320 from the VA Health Services Research and Development Service and by grant R01-MH078698-01 from the National Institute of Mental Health. The authors acknowledge substantial assistance from Ciaran Phibbs, Ph.D., and Mark Smith, Ph.D. The views expressed in this article are those of the authors and do not necessarily represent the views of the VA.
Dr. Olfson has received research support from Bristol-Myers Squibb, Eli Lilly and Company, AstraZeneca, and Ortho-McNeil Janssen Scientific Affairs. He has also worked as a consultant for Pfizer, Janssen, Bristol-Myers Squibb, and Eli Lilly and Company. The other authors report no competing interests.