Many patients with depression respond after a switch of antidepressant medications (
10 ). In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, one-quarter of depressed patients who derived little benefit from a selective serotonin reuptake inhibitor (SSRI) achieved remission after switching to one of several different antidepressants (
11 ). In an earlier study, more than half of patients whose depression persisted after an imipramine trial benefited from switching to sertraline (
12 ). Switching medications is also recommended for patients who have difficulty tolerating an initial antidepressant trial (
5,
6 ). In one study, adverse effects were responsible for a substantial proportion (43%) of early switches and discontinuations of SSRIs (
13 ).
In this article we describe rates of early antidepressant switching among adults initiating antidepressants for the treatment of a depressive disorder. The analyses focused on patients who adhered to antidepressant therapy during the first three months of treatment. We also examined the frequency with which patients switched within and between major classes of antidepressants. We hope that these analyses provide a foundation for future studies of antidepressant switching among patients who are nonadherent with medications early in the course of treatment.
Methods
Data source
Enrollment, service claims, and pharmacy data files from PharMetrics for the period 2001–2006 served as the source of data. This database contains standardized prescription, service claims, and enrollment information from more than 75 managed care organizations that collectively cover more than 55 million persons. Enrollment data include information on patients' age, sex, and periods of service eligibility. Claims data include diagnostic and provider information for all provided inpatient and outpatient services. Prescription claims include name of the medication filled, quantity of units dispensed, days of supply, and National Drug Code.
All study procedures were approved by the institutional review board of the New York State Psychiatric Institute. The data set includes no personal identifying information.
Sample selection
Patients treated for depressive disorders (N=56,521) with antidepressants were selected in several stages. First, patients were selected for continuous enrollment for ≥90 days before and ≥90 days after an antidepressant prescription and for absence of inpatient treatment during this period. Second, patients were required to have one or more service claims for a depressive disorder (
ICD-9-CM codes 296.2, 296.3, 311, or 300.4) within 30 days before or after the first antidepressant prescription. Third, patients were required to start one antidepressant; 723 patients who started two or more antidepressants on the same day were excluded from the sample. Fourth, supply of the first antidepressant prescription was required to be for ≤30 days, and patients were required to have received treatment with one or more antidepressants for ≥72 of the first 90 days after the first antidepressant prescription. This criterion focused the analysis on patients who met conventional standards for antidepressant adherence—a medication possession ratio of ≥.80 (
18,
19 ) during acute and early continuation phases of treatment (
20 ). It eliminated a large number of patients (N=63,630). Fifth, we excluded patients with any prescriptions for antidepressants, antipsychotics, mood stabilizers, anxiolytics and hypnotics, or stimulants or who had received electroconvulsive therapy during the 90 days before the first antidepressant prescription. Sixth, patients with one or more claims for bipolar disorder, schizophrenia, or a related psychosis (
ICD-9-CM codes 295–299, excluding codes 296.2 and 296.3) during the 90 days before or after the first antidepressant prescription claim were excluded. Seventh, patients who were younger than age 18 or older than age 75 were excluded. Eighth, the few patients in the data set with a public source of insurance were excluded.
The first antidepressant prescription was required to be for an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), a TCA, bupropion, mirtazapine, or trazodone. SSRIs included fluoxetine, paroxetine, sertraline, escitalopram, and citalopram. SNRIs included venlafaxine and duloxetine. TCAs included amitriptyline, doxepin, imipramine, maprotiline, protriptyline, and trimipramine. A residual group of other antidepressants included mirtazapine, bupropion, and trazodone.
We excluded patients starting nefazodone, which was withdrawn from the U.S. market in 2004; monoamine oxidase inhibitors (isocarboxazid, phenelzine, and tranylcypromine), which are not commonly prescribed; antidepressants not approved for the treatment of depression (clomipramine and fluvoxamine); and amoxapine, which has antipsychotic properties (
21 ). Because some antidepressants are sedating and may be used as hypnotics at low doses, we excluded trazodone (<150 mg per day) (
22 ), doxepin (≤50 mg per day) (
23 ), amitriptyline (≤75 mg per day) (
24 ), trimipramine (≤100 mg per day) (
25 ), and mirtazapine (≤15 mg per day) (
26 ).
Definitions of variables
Selected patients were dichotomously classified by antidepressant switching status during the first 90 days of antidepressant treatment. An antidepressant switch was defined as prescription of a second (different) antidepressant within 90 days of the first antidepressant prescription in which the days of supply of the second antidepressant extended for at least 15 days beyond the supply of the last prescription of the first antidepressant. A 15-day period was used to distinguish antidepressant switches from concurrent treatment with two antidepressants. Treatment with two concurrent antidepressants is not uncommon (
27 ) and has been recommended in some clinical situations (
28 ).
We also examined several demographic and clinical characteristics of patients: age (18–35, 36–55, and 56–75 years), sex, and specialty of the physician prescribing the first antidepressant. Physician specialty was classified as psychiatry, primary care (general practice, family practice, internal medicine, pediatrics and adolescent medicine, and obstetrics-gynecology), or other medical specialties. Patients were further classified by whether the first medical service claim for a depressive disorder was dysthymia ( ICD-9-CM code 300.4), depression not otherwise specified (code 311), or major depression (code 296.2 or 296.3), and the last category was subclassified as mild or moderate (code 296.21, 296.31, 296.22, or 296.32), severe (code 296.23, 296.33, 296.24, or 296.34), or unknown ( ICD-9-CM codes with no fifth digit).
One or more service claims for the treatment of a substance use disorder (
ICD-9-CM code 291, 292, or 303–305) or anxiety disorder (code 300.0, 300.2, 300.3, or 308.3) during the 90 days before the first antidepressant prescription defined treatment of these comorbid disorders. One or more claims during this period for any of the major medical illnesses in the Charlson Comorbidity Index (
29,
30 ) classified patients as having received treatment for a serious general medical illness.
A dichotomous variable represented use of emergency services for the treatment of a mental disorder (
ICD-9-CM codes 290–319) during the 90 days before the first antidepressant prescription. A dichotomous variable also represented any psychotherapy visit (Current Procedural Terminology codes 90804–90829, 90841–90847, 90849, 90853, 90855, 90857, 90875, and 90876) during this period. The Antidepressant Treatment History Form (
31 ) classified patients by initial antidepressant dose on a 4-point scale (1, lowest; 4, highest). Four copayment groups (U.S. $0–$10, $11–$20, $21–$30, or ≥$31) defined patients' out-of-pocket costs for the first antidepressant prescription.
Analytical approach
The rate of antidepressant switching was determined during the 90-day study period both overall and by patient age group, sex, physician specialty, depression type, comorbid mental and general medical disorder, emergency and inpatient mental health service use, antidepressant class, initial antidepressant dose, and antidepressant prescription copayment group. Differences in proportions were used to examine the strength of associations between each of these preswitching variables and antidepressant switching status. These analyses were designed to test the hypothesis that antidepressant switching is directly related to psychiatric illness severity as measured by treatment of major depressive disorder compared with other depression disorders, treatment by a psychiatrist, treatment of comorbid mental disorders, and recent emergency and inpatient mental health service use.
A logistic regression was performed in which the dependent variable was antidepressant switching status and each of the covariates served as independent variables. Similar analyses were performed by class of the patient's first antidepressant (SSRI, SNRI, TCA, and other) and by individual antidepressants within these classes. These regressions estimated the strength of independent associations between each covariate and antidepressant switching status and controlled for the demographic and clinical covariates. Because of the low frequency of first prescriptions for each TCA, separate analyses were not performed for each TCA.
Among patients who switched antidepressants, bivariate and multivariate analyses examined associations between individual antidepressants and switching status during the first 30 days of antidepressant treatment. The proportions of patients switching antidepressants and the surrounding confidence intervals were determined. A set of chi square analyses compared the observed distribution of antidepressant switches between classes with the distribution we expected on the basis of the initial antidepressant class distributions.
We considered group differences with a two-tailed alpha of <.01 to be statistically significant and those with an adjusted odds ratio of ≥1.3 or ≤.7 to be potentially substantial from a clinical or policy perspective.
Results
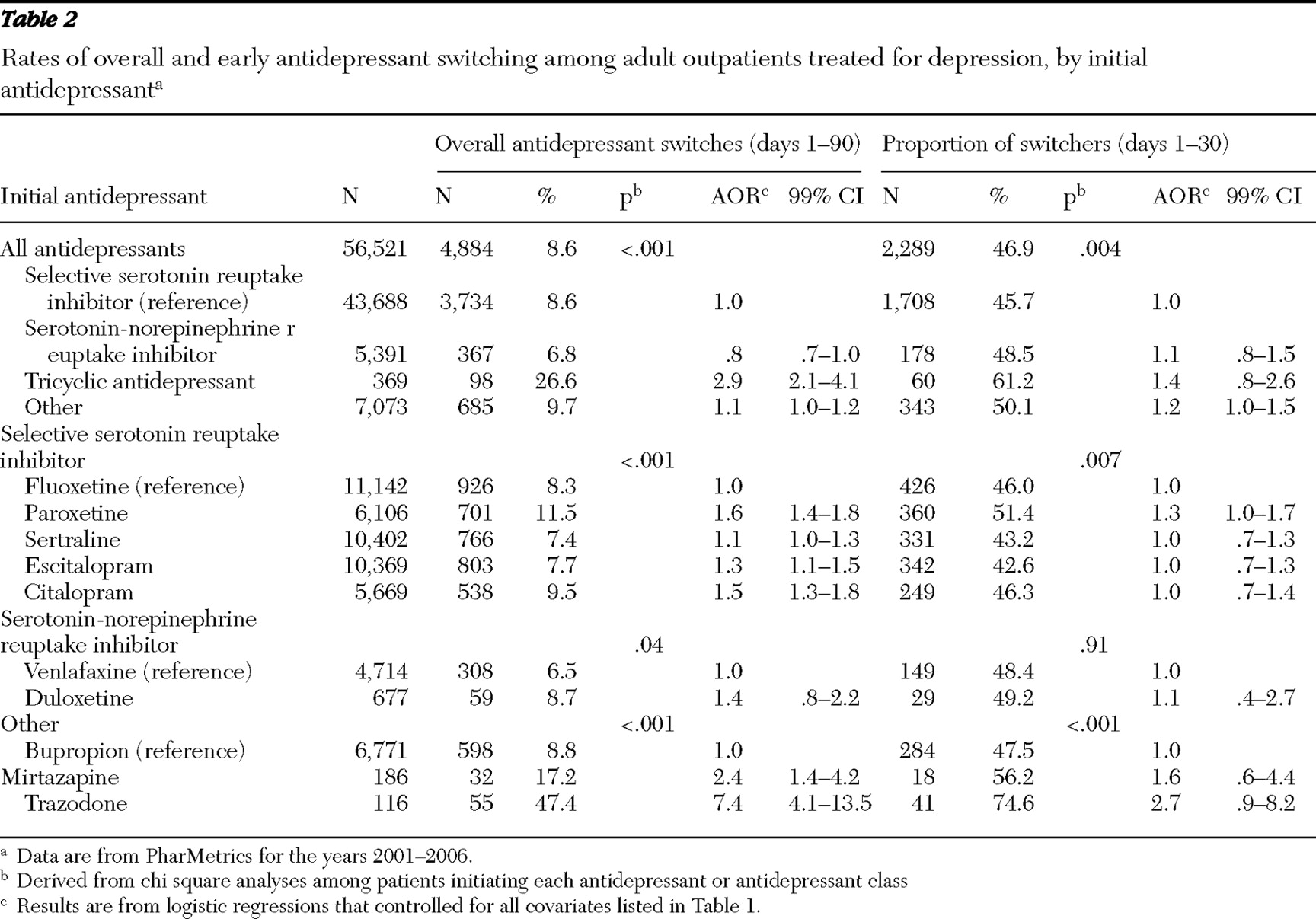
During the first 90 days of antidepressant treatment, 8.6% (N=4,884) of study patients switched antidepressants, and an additional 2.4% (N=1,333) added a second antidepressant without stopping the first antidepressant.
In unadjusted analyses, the probability of switching antidepressants significantly varied across patients' age, sex, and physician specialty, with higher switching rates among younger patients, males, and patients treated by psychiatrists, as well as among those treated for major depressive disorder, especially for severe episodes, which were also a strong predictor of antidepressant switching. In multivariate analyses, statistically significant and potentially substantial associations were observed between antidepressant switching and major depression, treatment in an emergency department for a mental disorder, and initiation of a TCA. Patients starting on very low doses of antidepressants were significantly more likely to switch antidepressants than those who started on a higher, but not the highest, dose (
Table 1 ). Antidepressant switching also varied by antidepressant class; the proportion of switches was highest for patients prescribed TCAs and lowest for patients prescribed SNRIs.
Some of these associations were observed in a model that controlled for all of the covariates (
Table 1 ). However, physician specialty, treatment of comorbid anxiety or substance use disorders, and prescription copayment were not significantly associated with switching after we controlled for the covariates.
After analyses controlled for background characteristics, starts on paroxetine, escitalopram, or citalopram, compared with starts on fluoxetine, significantly predicted a switch in antidepressants. Initiating mirtazapine or trazodone (versus bupropion) also significantly predicted antidepressant switching (
Table 2 ).
Nearly one-half (46.9%) of patients who switched antidepressants during the first 90 days of treatment did so during the first 30 days (
Table 2 ). Among patients who switched antidepressants, early switching was proportionately more common among patients starting TCAs than among patients starting other antidepressants, but this difference was not significant after we controlled for several potentially confounding factors. Within antidepressant groups, no individual antidepressants predicted switching during the first 30 days after we controlled for background characteristics (
Table 2 ).
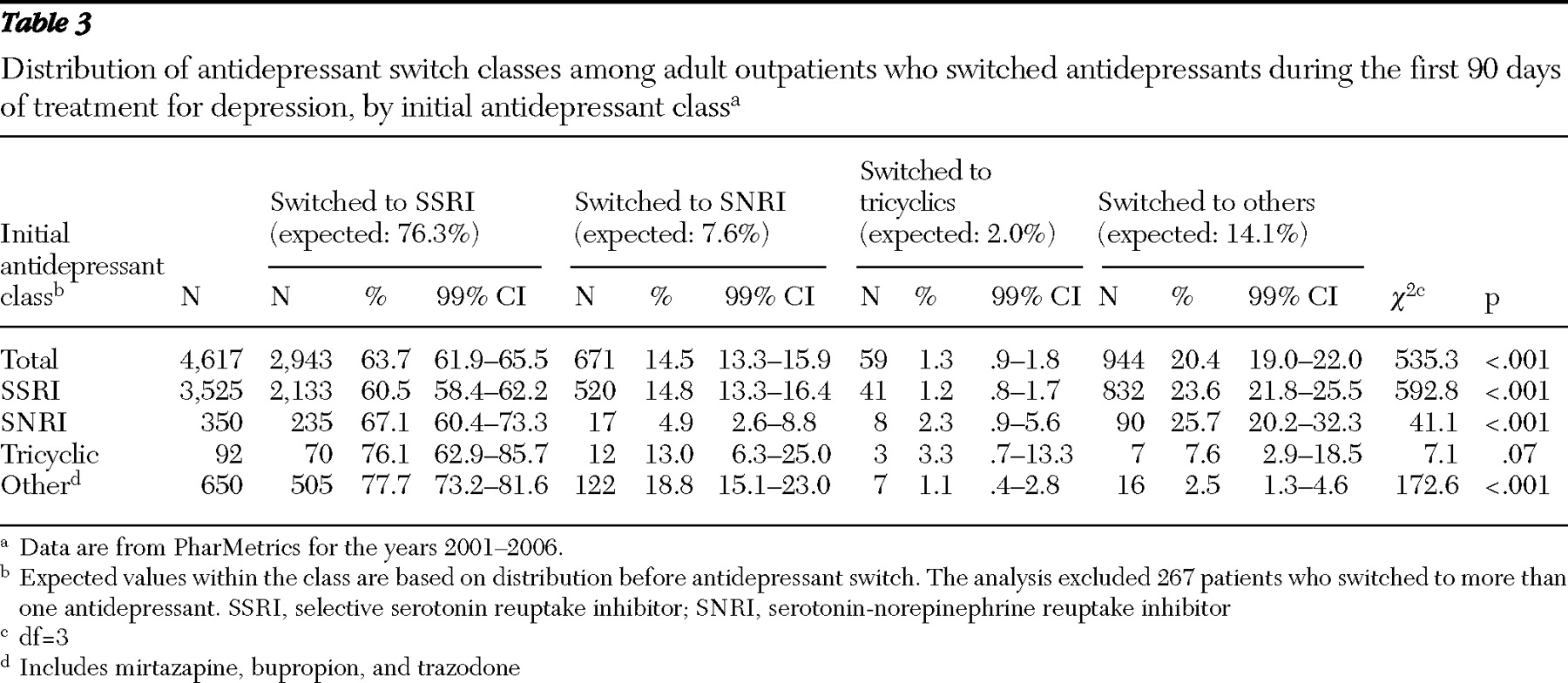
Most patients who switched antidepressants switched to SSRIs. In descending frequency, SSRIs were followed by the residual "other" antidepressants (mirtazapine, bupropion, or trazodone), SNRIs, and TCAs (
Table 3 ). For all patients but those starting with TCAs, the observed switching pattern significantly departed from the pattern expected on the basis of the patient's initial class of antidepressant. A smaller percentage of patients than expected switched from one SSRI to another SSRI (60.5% versus 76.3%). Similar significant departures from expected distributions were observed for several other switches (
Table 3 ).
In a post hoc analysis limited to patients who switched antidepressants, a significantly larger proportion of patients switched to antidepressants requiring a lower copayment (N=1,436, 33.5%; 99% confidence interval [CI]=31.7%–35.4%) than a higher copayment (N=838, 19.6%; 99% CI=18.1%–21.2%; data not shown).
Discussion
Antidepressant switching signifies a general lack of satisfaction with medication tolerability or efficacy from the perspective of the patient or physician. Approximately 8.6% of medication-adherent patients with depression who started with one antidepressant switched to a different antidepressant during the first three months of treatment. Another 2.4% of patients added a second antidepressant medication during this period without discontinuing the first antidepressant. Methodological differences between studies complicate direct comparisons of these figures with previous research (
32,
33,
34 ). In one earlier analysis, however, 13.5% of adult primary care patients with depression who started with SSRIs switched antidepressants during the three months after their first antidepressant prescription (
33 ). Switching (and adding) antidepressants, therefore, is less frequent than would be expected when considering the high rate of incomplete symptom resolution in clinical trials (
1,
2,
3 ). This gap suggests that many patients and clinicians accept incomplete treatment response rather than risk switching or adding antidepressants.
Antidepressant switching was associated with markers of illness severity. Patients treated by psychiatrists, those diagnosed as having more severe types of depression, those recently treated in an emergency department for a mental disorder, and those treated for a comorbid substance use disorder were all more likely to switch antidepressants. These associations are consistent with the hypothesis that clinical severity or complexity tends to increase the likelihood of incomplete antidepressant treatment response or problems with medication tolerability that in turn increase the likelihood of antidepressant switching.
Switching rates differed by initial antidepressant selection. Patients starting TCAs were more likely than those starting other antidepressants to switch antidepressants. Consistent with this observation, one long-term randomized effectiveness trial reported that patients assigned desipramine or imipramine were far more likely to switch antidepressants than those assigned fluoxetine (
17,
35 ). The rate of switching among patients starting with TCAs in this study (26.6%) roughly resembles that of the TCA group in that trial (35%) (
17 ). Patients in this study who started treatment with trazodone were also very likely to switch antidepressants. This finding is consistent with an earlier report of patients treated with trazodone; they had a significantly higher switch rate than patients treated with fluoxetine (
36 ).
The antidepressants with high switching rates share sedation as a frequent side effect. Sedation is common with mirtazapine, trazodone, and TCAs—except desipramine and imipramine—but is less common with the other antidepressants in this study (
36 ). Pharmacovigilance studies have revealed that drowsiness or sedation accounts for most adverse drug reactions during the first month of treatment with mirtazapine (
37 ). Sedation is also reported more than twice as frequently with trazodone compared with fluoxetine (
38 ). Without detailed clinical information concerning patient-reported reasons for antidepressant discontinuation and switching, it is not possible to test the hypothesis that sedation contributes to the observed pattern of antidepressant switching. For example, depressed patients with transient disturbed sleep may be preferentially started on short-term regimens of sedating antidepressants.
Among the SSRIs, sertraline was associated with the highest rate of antidepressant switching, although subtle clinical selection effects cannot be excluded from an explanation for this pattern. A nine-month randomized, controlled effectiveness trial of adult primary care patients with depression reported numerically higher rates of antidepressant switching among patients experimentally assigned to paroxetine (22%) versus sertraline (17%) or fluoxetine (14%) (
2 ). Although these group differences did not reach statistical significance in the clinical trial, it is possible that differences in the side-effect profiles of the SSRIs (
39 ) contributed to observed differences in switching rates.
There is evidence from various clinical settings that depressive disorders are commonly treated with inadequate doses of antidepressants (
40,
41,
42 ). We found that lower initial antidepressant dosing was related to subsequent switching. Low initial dosing might have contributed to suboptimal early treatment response and thereby increased the likelihood of antidepressant switching.
In an effort to reduce pharmacy costs, many health care plans have implemented multitiered formularies that progressively increase copayment for generic medications, preferred branded medications, and nonpreferred branded medications (
43 ). Lower copayments have been found to reduce the risk of antidepressant discontinuation (
44 ). In this study, which excluded patients who discontinued antidepressants, lower antidepressant copayment was related to a slightly increased risk of switching antidepressants, although not after adjustment for background characteristics, including antidepressant class, which was highly correlated with copayment. Among patients who switched antidepressants, there was a tendency to select antidepressants with a lower copayment, as patients and physicians presumably sought to reduce patient out-of-pocket costs.
Theoretical recommendations (
45,
46 ) and practitioner surveys (
16,
17 ) support the concept of switching across rather than within antidepressant classes. Preferential switching across antidepressants is not, however, supported by findings from the STAR*D. In this trial, there was no significant group difference in remission rates among adult depressed patients unsuccessfully treated with citalopram (an SSRI) and then randomly switched to sertraline (an SSRI), venlafaxine (an SNRI), or bupropion (other type of antidepressant) (
11 ). In community practice, higher than overall expected switching to SNRIs or to buproprion, mirtazapine, or trazodone suggests that these medications tend to be used as second-line agents.
This study had several limitations. First, no information was available concerning reasons for switching antidepressants or the clinical appropriateness of the switches. Patients may switch medications because of incomplete treatment response, medication tolerability problems, cost, patient or physician preference, or other reasons. Because the results concerned switching as a whole, they may conceal important heterogeneity in determinants of switching for different reasons. Second, associations of patient and clinical characteristics with antidepressant switching may be confounded by important unmeasured variables. If, for example, patients who were given a prescription for mirtazapine were significantly more treatment resistant as a group than patients who were given a prescription for bupropion, it might help to account for the observed difference in switching rates. Third, information was not available concerning aspects of antidepressant access, such as formulary restrictions, prior authorization requirements, "fail-first" policies, prescription rebates, availability of samples, or other factors that may influence antidepressant choice and switching patterns. Fourth, the data set did not capture antidepressant switches that involved antidepressant samples or other out-of-plan antidepressant acquisition. Fifth, the samples were so large that some statistically significant differences may have little clinical significance.
Sixth, the study focused on factors associated with switching among initially adherent patients who switched antidepressant medications during the first 90 days of treatment. Other factors may come into play earlier or later in the course of treatment. A large proportion of patients starting antidepressant medications discontinue the medications during the first three months of treatment. Antidepressant switching among partially medication-adherent patients is an important topic that deserves study.
Finally, this study concerned privately insured patients, and the results may not generalize to publicly insured or uninsured patients. In view of these limitations, prospective research might usefully assess depressed adults and their physicians to characterize in greater clinical detail the decision-making processes that govern antidepressant switching in community treatment.