Study population
The study was part of a larger-scale antidepressant research project, details of which have been reported elsewhere (
10). The study population consisted of adults age 18 and older who filled no antidepressant prescriptions during the last nine months of 1993 and subsequently filled at least one prescription for either a tricyclic or an SSRI during the first three months of 1994. The purpose of excluding patients who had used antidepressants in the previous nine months was to yield a population of first-time or episodic users, rather than chronic users. The first of the antidepressant prescriptions served as an index date, denoting the start of treatment. Patients were followed from the index date through April 30, 1995. The observation period before the index date was nine to 12 months, and after the index date it was 13 to 16 months.
Patients who were not continuously eligible to receive prescription benefits through ESI/VRx for the entire observation period (April 1, 1993, through April 30, 1995) were excluded. Also excluded were patients enrolled in either HMO or indemnity plans that restricted use of particular antidepressant products, and patients who used antidepressants in other drug classes, such as trazodone, buproprion, and venlafaxine. Patients taking more than one antidepressant drug because they were being switched to another drug or because their treatment was being augmented were included, both to maximize the study's external validity and because sensitivity analyses produced the same findings when data for these patients were excluded. However, patients who filled prescriptions for two different antidepressants on the index date were excluded.
The previously reported size of the resulting sample was 4,252 (
10). However, the analyses reported in this paper include only a subset of 3,101 patients for whom prescriber information was available for all antidepressant claims.
Analytic procedures
Assessment of the course of antidepressant treatment included measures of length of treatment and daily dosages. They were calculated using data fields that are standardized on prescription claims nationwide. For each claim, the pharmacist designates a days'-supply figure that indicates how long the medication will last if taken as prescribed. The sum of the fill date plus the days'-supply figure equals the expected depletion date. Length of treatment was defined as the difference (number of months) between the index date and the last depletion date observed before the end of the study period on April 30, 1995. The prescribed daily dosage was calculated as the number of milligrams dispensed (number of pills times strength per pill) divided by the days' supply.
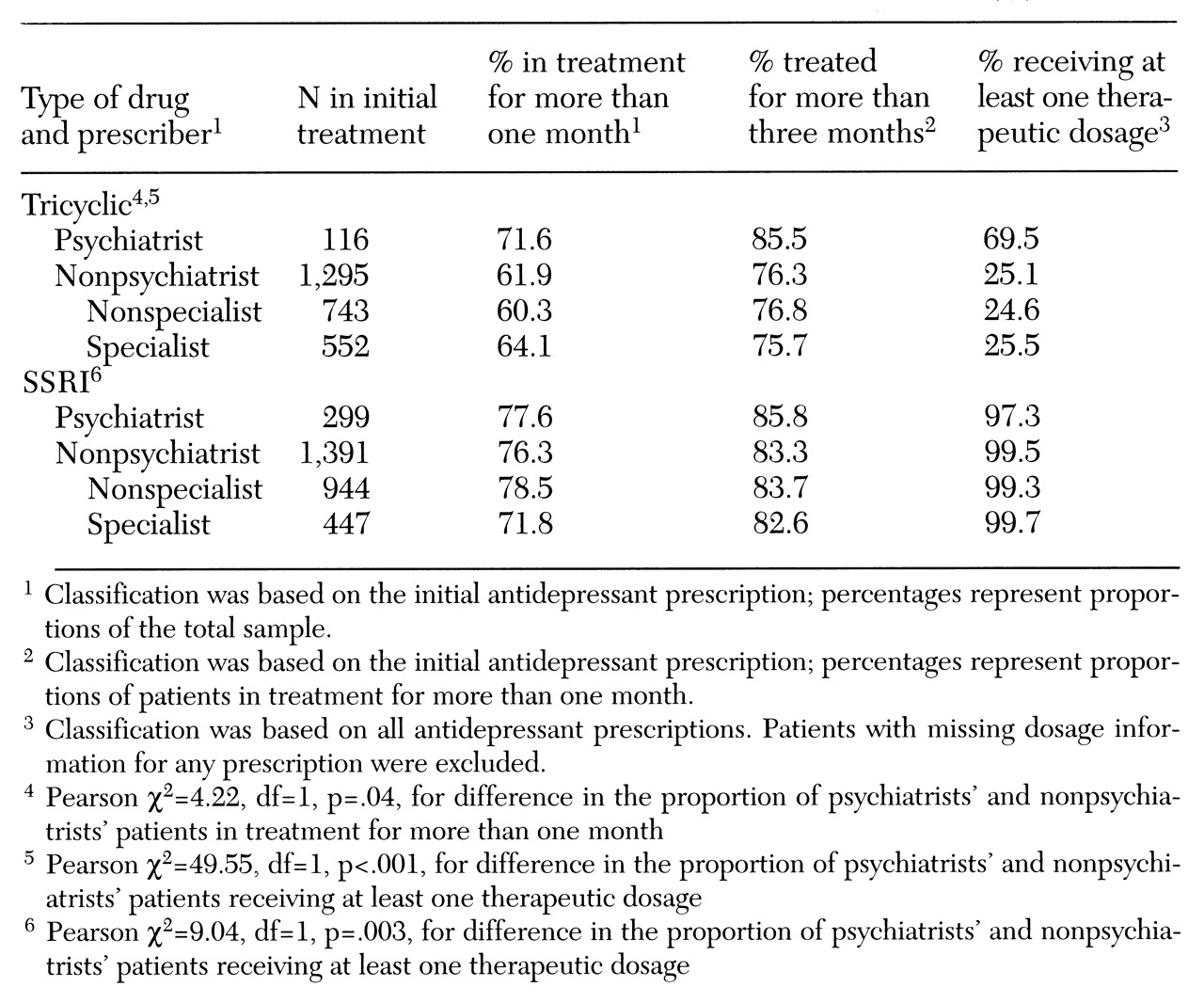
The first dependent variable was termination of treatment on or before one month, defined as a length of treatment less than or equal to 30 days. The second dependent variable, subtherapeutic dosing, was defined as a failure to receive at least one prescribed dosage meeting or exceeding therapeutic dosing standards. To distinguish therapeutic from subtherapeutic dosages of tricyclics, standards originally developed for the Medical Outcomes Study were used (
5). For SSRIs, standards were drawn from the usual dosages listed in the
Physicians' Desk Reference (
13).
The resulting thresholds for patients age 60 or younger were 100 mg daily for amitriptyline, desipramine, doxepin, and imipramine; 75 mg daily for nortriptyline; 50 mg daily for sertraline; and 20 mg daily for paroxetine and fluoxetine. Standards for patients age 61 or older were lower: 75 mg daily for amitriptyline, desipramine, doxepin, and imipramine; 50 mg daily for nortriptyline; 25 mg daily for sertraline; and 10 mg daily for paroxetine and fluoxetine. To allow time for titration, the second dependent variable—subtherapeutic dosing—was measured only for patients who were treated for at least three months with one drug. For example, a patient treated six weeks with one drug and six weeks with another would be excluded from this analysis.
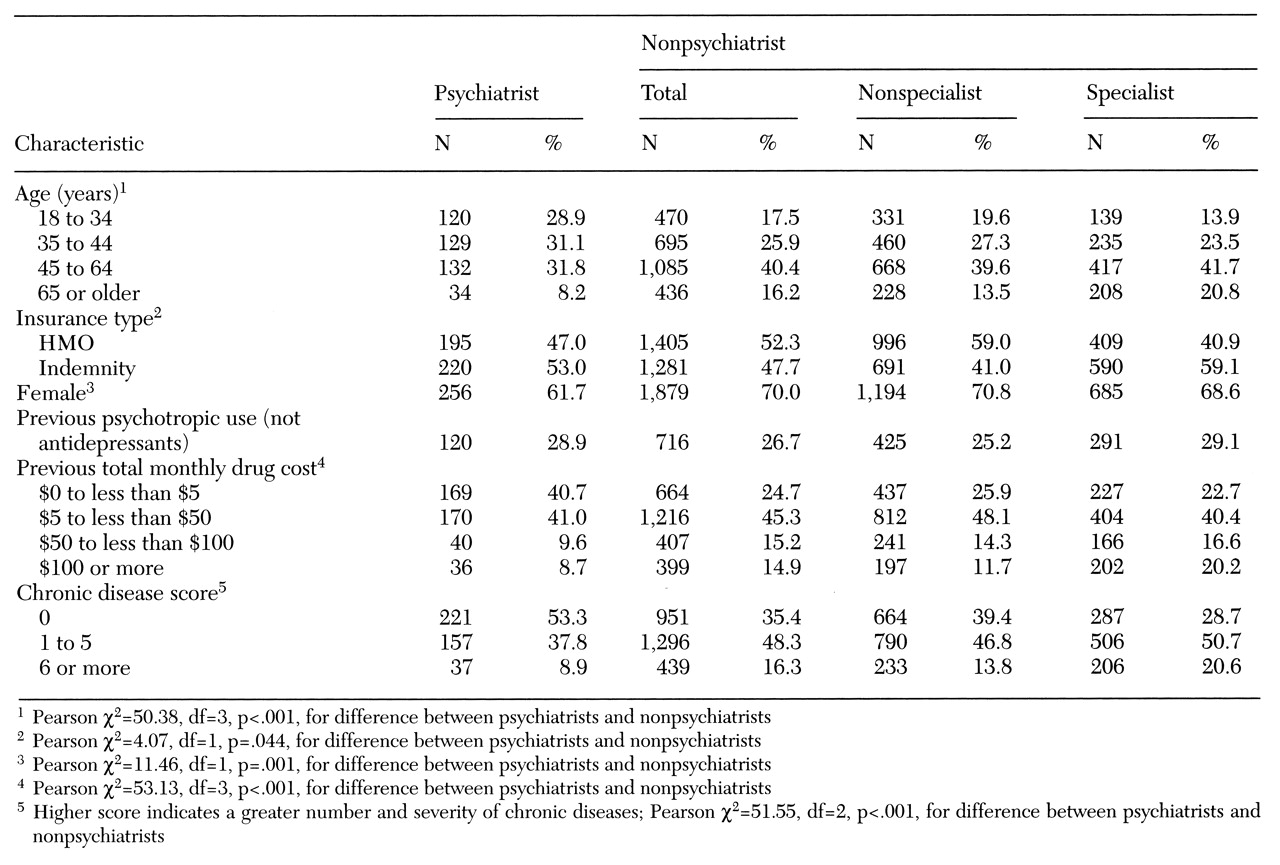
Independent variables included the patient's age on January 1, 1994; gender; type of insurance coverage (HMO or indemnity plan); and two measures of use of nonantidepressant medications during the nine to 12 months before antidepressant therapy. These measures were whether the patient used psychotropic drugs other than antidepressants and the monthly cost (average wholesale price) for all drugs (not just psychotropics).
A chronic disease score was calculated for the time period from six months before to six months after the index date, using an algorithm based solely on pharmacy claims data (
14). Because pharmacy claims contain no diagnostic information, the algorithm imputes diagnoses from drug utilization incorporating measures of cardiac disease, respiratory illness, rheumatic disease, cancer, and other conditions. The summary chronic disease score reflects both the number and severity of chronic conditions, is predictive of subsequent hospitalization and mortality, and correlates with physicians' ratings of patients' disease severity (
14).
Data about the prescriber's specialty were obtained by matching information in the American Medical Association national database to prescribers' Drug Enforcement Agency numbers, names, and addresses. For analyses of termination at one month, the physician who wrote the index antidepressant prescription was classified as a nonspecialist, such as a general practitioner, family practitioner, or general internal medicine practitioner; a nonpsychiatric specialist, such as a cardiologist, gastroenterologist, or neurologist; or a psychiatrist.
For analyses of subtherapeutic dosing, the same specialty classifications were used, but analyses were based on all antidepressant prescriptions rather than on the index prescription only. Patients were classified into one of three groups—all prescriptions written by nonspecialists, at least one written by a specialist but none by a psychiatrist, or a least one written by a psychiatrist.
Analyses were performed using SPSS for Windows NT, version 7.5. In descriptive analyses, proportional differences were tested for statistical significance using Pearson's chi square test. Cost and age differences were tested using SPSS's medians test, which is nonparametric. Statistical tests of prescriber type compared psychiatrists and nonpsychiatrists, instead of comparing all three types. The rationale for this approach was that the study's primary objective was to compare psychiatrists with other providers, not to compare different types of nonpsychiatric providers.
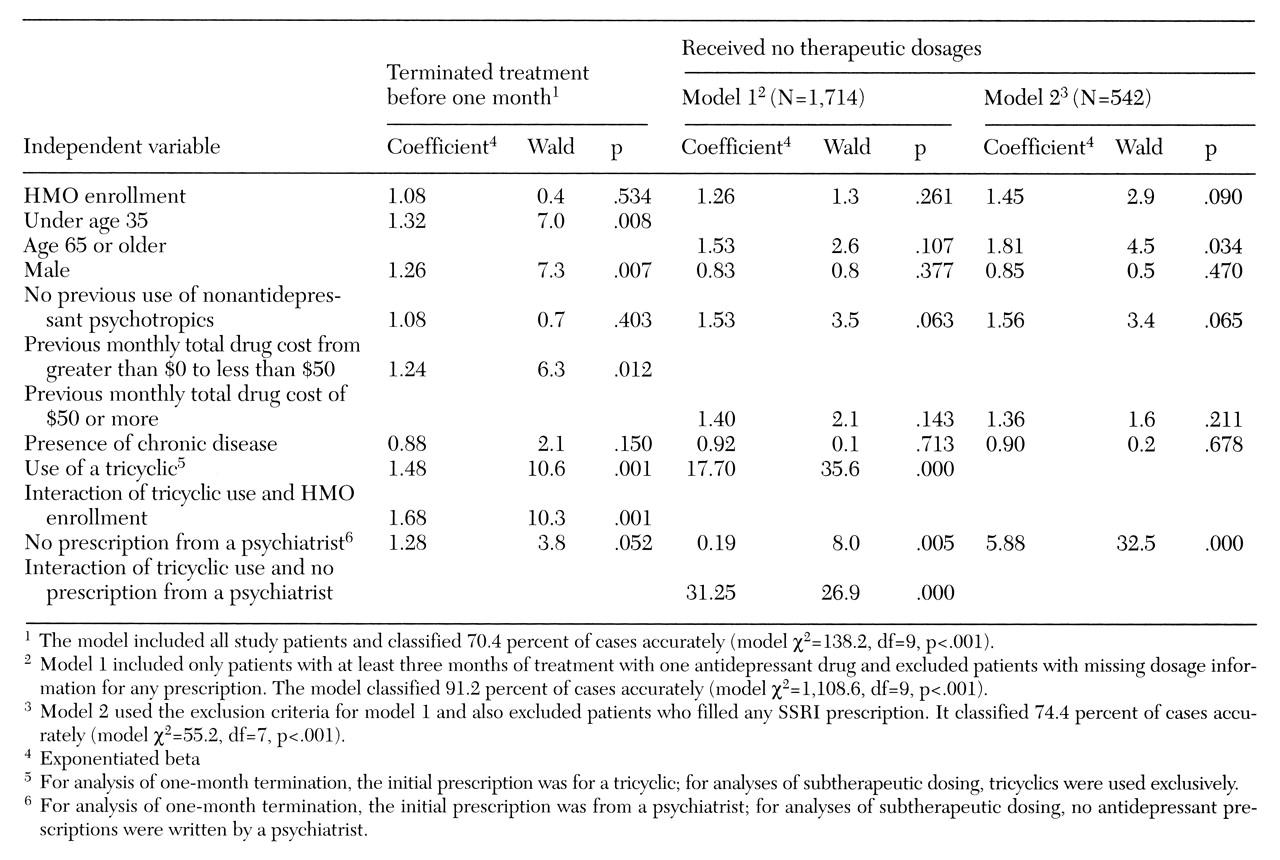
Logistic regression analyses were performed to assess the impact of the prescriber's specialty on duration and dosage, controlling for the other independent variables. In these analyses, change in -2 times log likelihood, a goodness-of-fit measure with a chi square distribution, was used to test the models. Coefficient testing was performed using the Wald statistic. The significance level was set at .05.