The psychiatric family study literature generally supports the notion of independent intergenerational transmission of schizophrenia and affective disorders (
1–
3), although some authors assert that these disorders share a common genetic etiology (
4,
5). Depressive symptoms occur frequently in patients with schizophrenia and often represent substantial clinical management challenges. We tested the hypothesis that the affective components sometimes observed in schizophrenic patients are related to a familially transmitted liability to affective disorders. Given the evidence that major affective disorders are transmitted familially, it is plausible that affective symptoms in schizophrenia may also be the result of a familial liability to affective disorders. Preliminary evidence from a study that had a less rigorous method than the present study (
6) suggests that affective components of schizophrenia are related to affective illness in the biological relatives of schizophrenic patients. This hypothesis contrasts with the view that affective symptoms, such as depressive symptoms, during the course of a schizophrenic disorder are part of the process of recovery from a schizophrenic episode (
7).
The present study combined family study and family history methods to assess the frequency of affective and schizophrenic disorders in the first- and second-degree relatives of patients with schizophrenia. First-degree relatives, for the most part, were directly interviewed (family study method), while the family history method was used to characterize the first-degree relatives who were not interviewed and all second-degree relatives. We examined the relation between measures of depressive symptoms in the probands and the aggregation of unipolar affective illness in the first- and second-degree family members. The probands were selected on the basis of an onset of a first psychotic episode less than 2 years before the index diagnosis (
8). The probands' symptoms were studied in the acutely psychotic and initial outpatient phases of their illness. Schizophrenic patients tend to show a wide range of psychotic symptoms, an episodic course, and, frequently, affective symptoms early in the course of illness. Furthermore, the role of any affective symptoms is often of clinical importance early in a psychotic illness. Thus, a recent-onset study group is well suited for examining the proposed relationships between affective symptoms in schizophrenic probands and psychiatric disorders in their relatives.
This study included several important methodological features that were recommended by Pope and Lipinski (
9). In this family study relatives' diagnoses were determined through direct personal interview in which systematic criteria were used by researchers blind to the diagnosis of the proband. As recommended by Eaves et al. (
10), the power of this type of family study was increased by treating the risk factors as continuous variables, measuring depression in both the probands and their relatives, and including second-degree relatives in the analyses.
Since the presence of a frank schizophrenic disorder tends to make parenthood less likely (
11), parents of schizophrenic probands, by virtue of their parent status, will tend to manifest only subtle forms of schizophrenia-related disorders. We therefore examined the full spectrum of disorders that apparently are genetically related to schizophrenia, including paranoid and schizotypal personality disorders (
12,
13). Thus, this study included a broader assessment of familial psychiatric illnesses related to schizophrenia than did many previous family studies of schizophrenia.
METHOD
Seventy patient probands with schizophrenia, diagnosed according to the DSM-III-R criteria, and their biological relatives served as subjects. All probands were receiving outpatient treatment at the UCLA Aftercare Research Program and were part of a large longitudinal study of the early phase of schizophrenia called the Developmental Processes in Schizophrenic Disorders project (
8,
14). To enter the longitudinal study, the patient's first psychotic episode had to have occurred less than 2 years before contact with the project. Family history of psychiatric illness was not considered in the selection of probands. A complete description of the selection criteria for this study group can be found in an article by Nuechterlein et al. (
8). The proband group consisted of 60 men and 10 women who were relatively young (mean age=23.5 years, SD=4.7, range=18–44), whose parents were of average social class (rating of 2.8 on the Hollingshead scale [15], SD=1.2, range=1–5), and who had completed an average of 12.6 years of school (SD=2.0, range=8–16). Sixty-three probands were non-Hispanic Caucasian, three were Hispanic, one was Asian, and three were of mixed racial background. These probands represent the first 70 probands with a DSM-III-R diagnosis of adult-onset schizophrenia whose families were recruited for a family genetic study called the UCLA Family Members Study (K.H.N. and R.F.A., Principal Investigators).
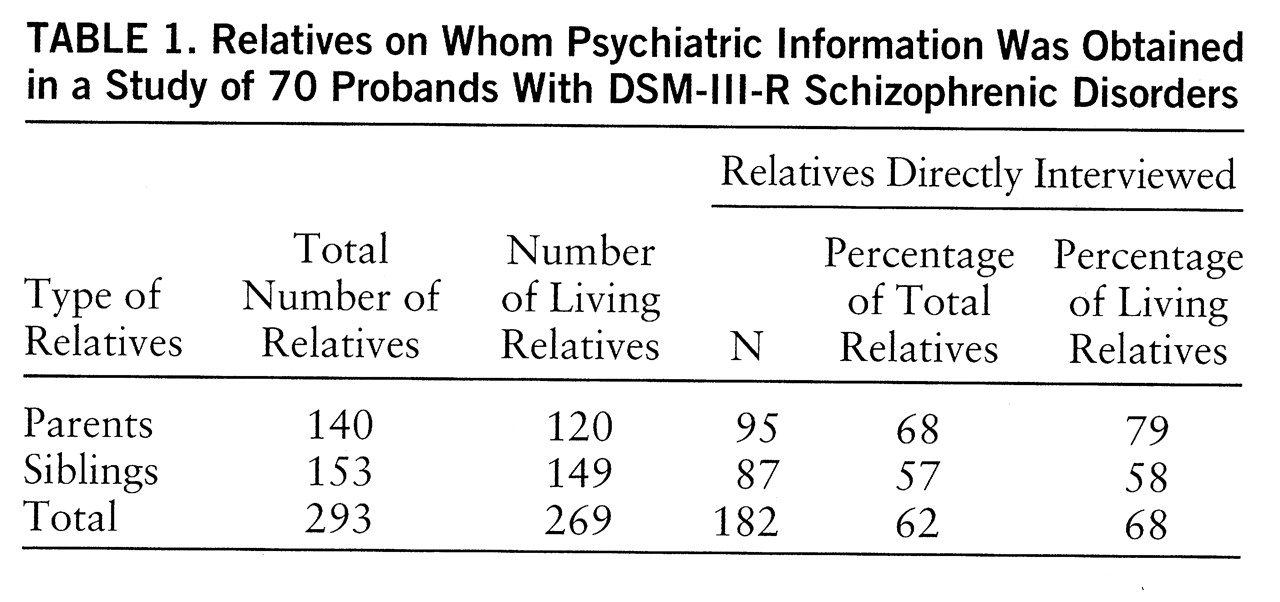
Psychiatric diagnostic information on 293 first-degree and 674 second-degree relatives of the probands was obtained from 182 directly interviewed first-degree relatives who participated in the UCLA Family Members Study, a large ongoing study of psychiatric disorders and of attentional functioning and information processing in relatives of psychiatric patients. The average age of the 182 directly interviewed first-degree relatives was 43.2 years (SD=15.2, range=17–75). The 80 male and 102 female first-degree relatives who participated had completed an average of 14.0 years of school (SD=2.5, range=4–20).
Table 1 gives further information regarding the relatives whose data were used in the study. After a complete description of the study to the subjects, written informed consent was obtained.
Measurement of Probands' Depressive Symptoms
Several measures of probands' symptoms were derived from data of the longitudinal study. The DSM-III-R symptoms of depression and mania were rated during the index psychotic episode with a version of the Present State Examination (PSE) (
16) that was expanded to assess the full range of symptoms for the Research Diagnostic Criteria (RDC) (
17) and DSM-III-R diagnoses of major affective disorders and schizophrenia. Following didactic instruction and practice interviews, interrater agreement was examined for ratings of nine videotapes of PSE interviews and of one live interview. The average percent agreement on individual symptoms rated present by the PSE trainer, who served as the criterion rater, was 88%. The average percent agreement for individual symptoms rated absent was 94%.
A dichotomous variable of “inpatient depression” was created by dividing the probands into two groups according to whether or not they met DSM-III-R criterion A (sufficient depressive symptoms) for a major depressive episode. All patients who showed inpatient depression according to this criterion had nonetheless been diagnosed as having DSM-III-R schizophrenia, as opposed to DSM-III-R schizoaffective disorder, because the affective symptoms were judged not to have played a prominent role in the overall psychotic episode. An affective syndrome was judged not to be prominent if it was brief relative to the total duration of psychotic symptoms during the episode. Also taken into consideration were the severity of the affective symptoms and whether pharmacologic agents were being used to treat the mood disturbance.
Since these probands had an average of only one manic symptom at the inpatient assessment, and none met the criteria for a DSM-III-R manic episode, manic symptoms were not examined.
The expanded Brief Psychiatric Rating Scale (BPRS) (
18) was used every 2 weeks to assess symptom levels of the 70 outpatients. The ratings on the BPRS were done by UCLA Aftercare Research Program staff, who were distinct from the UCLA Family Members Study staff and who were blind to the diagnoses of family members made by the latter staff. The BPRS raters completed intensive, specialized training using videotaped and live interviews (
19). They were required to show a median intraclass correlation with criterion ratings of at least 0.80 across items. The BPRS was administered every 2 weeks during the first outpatient year after stabilization of the patient on the standard starting dose of 12.5 mg of fluphenazine decanoate injected every 2 weeks. (Fifty-nine of the 62 DSM-III-R schizophrenic patient probands who completed the approximately 1-year follow-through period were administered the standard starting dose of 12.5 mg of fluphenazine decanoate every 2 weeks throughout most of the 1-year assessment period. Adjustments to this dosage, either upward or downward, were made in eight of these cases according to clinical need during the 1-year assessment period. Three patients were initially stabilized on somewhat lower doses because of uncomfortable side effects. Two patients were administered adjunctive antidepressant medication throughout the 1-year period, and another patient received antidepressant medication as well as lithium during the assessment year.)
A longitudinal 1-year index of depression was calculated for each proband by averaging the ratings on the expanded BPRS item “depressed mood” over the 1-year period. Sixty-two probands had an average of 21.5 (SD=3.9) separate BPRS administrations, covering approximately 43 weeks. The longitudinal depression index was not calculated for eight probands who had insufficient follow-through data. (These eight patients were administered the standard starting dose of 12.5 mg of fluphenazine decanoate at the beginning of the assessment period.) The patients were also grouped according to whether they experienced a clinically significant depressive period during the first outpatient year, as identified by BPRS criteria that paralleled those developed for significant psychotic exacerbation or relapse periods during the 1-year follow-through study phase (
8). A clinically significant depressive period was defined on the basis of ratings on the BPRS items of depressed mood, guilt, and suicidality. A significant depressive period after the absence of depressive symptoms involved 1) a rating of 5 (on a scale of 1–7) on one of the three items plus a 2-point increase on one of the other two items on any BPRS covering a 2-week period, 2) a rating of 5 on any of the three items for more than two consecutive 2-week BPRS periods, or 3) a rating of 6 or 7 on any one of these three items on any BPRS covering a 2-week period. A significant depressive period after persistent low-level depressive symptoms was defined as an increase to a rating of 6 or 7 points plus a 2-point increase on one of the other two depressive items. Changes in antipsychotic or antidepressant medication during this period were made on the basis of clinical considerations and were not contingent on these BPRS depression criteria.
Family Study and Family History Information
We attempted to interview directly all first-degree relatives aged 15 years or older. The interviewers were two doctoral-level clinical psychologists, two doctoral candidates in clinical psychology, and one master's-level research assistant with 10 years' experience in psychiatric research. The Modified DIS/PSE, which consists of a modified National Institute of Mental Health (NIMH) Diagnostic Interview Schedule (DIS) (
20) supplemented by additional PSE probes and ratings for psychotic symptoms, was used to assess the major RDC psychiatric diagnoses for the directly interviewed relatives. The RDC were used for axis I disorders for family members because they allow a subdivision of schizoaffective disorder into types that appear to be genetically related to schizophrenic and affective disorders. Thus, these subtypes could be assigned to the schizophrenia or affective disorder family history loadings used in this study (
21). For the UCLA Family Members Study, we modified the DIS to include a time line for affective and psychotic syndromes to aid in diagnostic decision making. These modifications ensured that all information necessary for determining RDC and DSM-III-R diagnostic status was elicited. Although the DIS has been used in a variety of epidemiologic studies and has been found to have good reliability and validity (
22,
23), our modifications were made to offset some concern about the sensitivity of this instrument for diagnosing schizophrenia. The use of the structured DIS interview ensured that no areas of psychopathology were overlooked, and the semistructured additional PSE probes and syndrome time line facilitated differential diagnosis of schizophrenia, schizoaffective disorder, and affective disorders. Also, only clinically skilled interviewers with additional specialized training in structured psychiatric interviewing were used.
The interviewers were trained to criterion level on the DIS by the same individual who trained the Los Angeles staff of the Epidemiologic Catchment Area project (
24). The training involved an intensive initial workshop, rating of sample DIS videotapes, “mock” interviews with friends and relatives, and, finally, interviews with adult inpatients and nonpatient volunteers. The interviewers were trained in PSE administration by the staff of the Diagnosis and Psychopathology Unit of the UCLA Clinical Research Center for Schizophrenia, who also trained the interviewers of the probands.
The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II) (
25) was used to assess five personality disorders, including two putative schizophrenia-spectrum disorders (paranoid and schizotypal). The training procedures and reliability assessment methods in the current study have been described elsewhere (
26). Audiotapes of 45 interviews were rated by separate interviewers to evaluate interrater reliability. Adequate interrater reliability, based on dimensional syndrome scores, was demonstrated for both paranoid and schizotypal personality disorders (intraclass correlations were 0.70 and 0.73, respectively), as well as for the three other personality disorders assessed in the relatives (
26). Reliable assessment of paranoid and schizotypal personality disorders has also been previously demonstrated with the SCID-II (
27).
Family history information on all first- and second-degree relatives (including directly interviewed relatives) was obtained from two adult informants in each family, usually the parents of the proband. Following the systematic generation of a genealogy diagram, family history information about major psychiatric illnesses was obtained with use of the NIMH Relative Psychiatric History Checklist Interview format (E.S. Gershon, unpublished). This format involved screening questions for the major psychiatric disorders, as well as added screening questions for the five personality disorders of interest. Any positive responses to these screening questions were followed up with more detailed probing. To keep the informant's task manageable, the screening questions were repeated separately for different sibships or for no more than 10 relatives at any one time. For these family history interviews, personality disorders were assessed with the SCID-II sections adapted to a third-person format.
As this investigation was part of a larger ongoing study involving diagnostic interviews with family members of probands with bipolar affective disorder, adult-onset schizophrenia, childhood-onset schizophrenia, attention deficit disorder, or no mental disorder, it was possible to keep the family interviewers blind to the proband's diagnosis. Typically, and whenever feasible, each first-degree relative was interviewed by a staff member who had not interviewed any of the other first-degree relatives within the same family. Since more than one individual covered in the family history interview might be assigned a psychiatric diagnosis, the diagnoses of relatives were by necessity sometimes made with knowledge of diagnoses assigned to other relatives (but not the proband) within the same genealogy. Information regarding other family members' psychiatric histories was not considered in making a family member's psychiatric diagnosis. To minimize further any potential bias in diagnostic judgments, the final consensus diagnosis of each family member who had any psychotic or quasipsychotic symptoms was made through a case presentation of all symptom information to the senior clinicians (K.H.N., R.F.A., and D.L.F.), who were blind to the diagnoses of the probands and all other family members.
Diagnoses of Relatives and Calculation of Family Loading
Consensus diagnoses were made at two levels. The first level was a consensus based on family history information from two family members and used the Family History RDC (FH-RDC) (
28) for the major clinical syndromes and DSM-III-R criteria for the personality disorders. Since the second-degree relatives were not directly interviewed, this first level of consensus diagnosis was the one used for these individuals. The second consensus level was derived from consideration of the family history and direct interview information, as well as psychiatric records if available, and was based on the regular RDC for the major clinical disorders and the DSM-III-R criteria for personality disorders. When direct interview information was available, the diagnoses from the second consensus level were used in the analyses. When direct interview information was not available, first-level consensus diagnoses were used in order to allow each first-degree relative to be represented. At both levels, discrepancies among raters or among the sources of information were reconciled in a meeting with senior investigators in which all decision makers were kept blind to the proband's diagnosis.
Family loadings were computed for each family for three groups of disorders: 1) unipolar-SAAD disorders (RDC definite or probable major depressive disorder; FH-RDC major depressive disorder; and RDC or FH-RDC schizoaffective disorder, mainly affective, depressed subtype); 2) bipolar-SAAM disorders (RDC or FH-RDC manic disorder; and RDC or FH-RDC schizoaffective disorder, mainly affective, manic subtype); and 3) schizophrenia-spectrum disorders (RDC or FH-RDC schizophrenia; RDC or FH-RDC schizoaffective disorder, mainly schizophrenic subtype; DSM-III-R paranoid personality disorder; and DSM-III-R schizotypal personality disorder).
To compute the family psychiatric history loadings, the rates of the three classes of disorders were first age-adjusted within each family for first- and second-degree relatives separately by using the Weinberg shorter method (
29). For lifetime morbid risk rates we used 15–39 years as the ages of risk for schizophrenia and schizophrenia-spectrum disorders and 15–59 years for all affective disorders. Individuals whose ages were within the ranges of the ages of risk contributed 0.5 to the denominator or Bezugsziffer (BZ), whereas relatives past the age of risk and individuals affected with the disorder in question contributed 1.0 to the BZ. To take the degree of genetic relationship between each relative and the proband into consideration in calculating a family psychiatric history loading, the morbid risk for the first-degree relatives in a family was multiplied by 0.50, which corresponds to the proportion of shared genes for a parent and proband and the average proportion of shared genes for the siblings. Similarly, the family morbid risk for the second-degree relatives was multiplied by 0.25, which corresponds to the average proportion of their shared genes. These two weighted morbid risk values were then summed to obtain the family loading. Thus, each family contributed three separate scores, one for each dimension of family psychiatric history loading. On infrequent occasions an individual received both a schizophrenia-spectrum diagnosis and an affective disorder diagnosis. For the purposes of the data analyses, these individuals were included in family loading calculations for both diagnostic categories. A natural log transformation of the family loading variables was used to normalize the distributions.
Statistical analyses were completed through four linear regression and logistic regression analyses in which the unipolar-SAAD family psychiatric history loading was used as the predictor variable. The bipolar-SAAM family history loading was not used as a predictor because there were insufficient cases of these disorders in the first- and second-degree relatives. If a significant predictive effect was observed for unipolar-SAAD family loading, the analytic plan was to rerun the regression analysis, forcing in the schizophrenia-spectrum disorder family loading first in order to assess the predictive value of the unipolar-SAAD family loading over and above that which could be accounted for by the variance associated with a schizophrenia-spectrum family history. When the two dimensions were included to evaluate the contribution of a family loading for unipolar affective disorder in the context of the schizophrenia-spectrum loading variable, only the affective disorder family history loading variables were hypothesized to predict affective features in probands. Therefore, the statistical tests for the unipolar-SAAD predictor rather than the overall model are the focus of interest (
30).
Because the interpretation of multiple regression analyses that use predictor variables showing high intercorrelations can be complicated, the correlation of the two family loading dimensions (unipolar-SAAD and schizophrenia-spectrum disorders) was computed to determine whether this would be a source of difficulty. The correlation was significant but quite low (Pearson's r=0.28, N=70, p<0.03), indicating some nonindependence of these two family loading dimensions but not enough to compromise the interpretation of the multiple regression analyses.
RESULTS
Twenty-one percent (N=15) of the 70 probands with schizophrenia met DSM-III-R criterion A (sufficient depressive symptoms) for a major depressive episode during the inpatient evaluation. In a logistic regression analysis, the unipolar-SAAD family psychiatric history loading did not significantly predict this proband inpatient DSM-III-R depressive symptom cluster. Thus, depressive syndromes that had been judged not to be a relatively brief part of the symptom picture during the index episode were not associated with family history of affective disorder.
The mean BPRS depressive mood ratings made during the patient's first outpatient year were the primary measure of outpatient depression. In a linear regression analysis, family loading for unipolar-SAAD disorders predicted significantly higher mean outpatient BPRS depression index scores (F=4.87, df=1,60, p<0.04). The regression analysis was computed again with the schizophrenia-spectrum family loading forced in before the unipolar-SAAD loading. The family loading for schizophrenia-spectrum disorders itself was not significantly predictive of higher mean outpatient BPRS depression index scores (F=0.94, df=1,59), but the unipolar-SAAD disorders continued to significantly predict BPRS outpatient depression level (F=5.66, df=1,59, p<0.03). The robustness of this association was confirmed by rerunning the regression analysis on 1,000 randomly generated subsets of the same data set to generate an empirical distribution of the regression test statistic. The statistic for this finding was significant for more than 99% of the statistical runs (Z=2.90, p<0.004). Thus, a family history of unipolar-SAAD disorders was associated, as hypothesized, with persistent low-to-moderate levels of depression as measured by the BPRS during the initial 1-year period after the index diagnosis. This was true even after accounting for the variance associated with a family history of schizophrenia-spectrum disorders.
Given the episodic nature of depression, an alternative definition was explored that involved the presence of clinically significant outpatient depressive periods, defined as BPRS significant exacerbations as described earlier. Fifteen (21%) of the patients experienced such a period of depression. Logistic regression analysis revealed that these discrete outpatient periods of clinically significant depressive symptoms were not predicted by the unipolar-SAAD family history variable.
To assess the influence on the findings of the Weinberg method of age correction used in the family loadings, the significant findings were reanalyzed with the use of family loadings that were not age-corrected. The significance levels of the age-corrected and non-age-corrected analyses were found to be essentially the same. While a more elaborate age-correction method, such as one based on survival analysis methods, might result in more precise estimates of morbid risk rates for each family, the choice of age-correction method did not appear to unduly influence the prediction of a proband's depressive symptoms based on the family psychiatric history loadings.
DISCUSSION
The results of this study provide support for the hypothesis that certain aspects of depression in schizophrenia are associated with a family history of affective illness. The mean outpatient depression level was significantly related to a family history of unipolar affective illness. Relatively brief periods of depressive symptoms during the acute inpatient psychotic episode at entry into the study were not associated with a family history of affective disorders. The positive finding is consistent with results of a study by Kendler and Hays (
6), which showed that a family history of affective disorders was related to the presence of affective symptoms in patients with schizophrenia. In that study, a family history of unipolar affective illness was related to a depressive aftermath during the outpatient follow-up period, and a family history of bipolar disorder was related to prominent depression in the prodromal period. The findings of the present study and those of Kendler and Hays are not consistent with the common view that depression in schizophrenia is solely an aspect of schizophrenia itself, such as a reaction to, or a part of, the recovery from a psychotic episode (
7).
Our findings suggest that the liability to affective disorders results in increased levels of outpatient depression. It is possible that this affective liability does not contribute to the psychotic process per se but colors the nature of the disorder such that it includes greater affective symptom components. This parallels the view put forth by Pogue-Geile and Harrow (
31) as a possible explanation for the occurrence of negative symptoms in schizophrenia, whereby the negative syndrome might be the result of other independent but co-occurring factors, such as low intelligence, that modify the schizophrenic outcome to include negative symptoms.
Our findings indicate that relatively brief periods of depressive symptoms during psychotic episodes are not related to a family history of depressive disorder and, thus, suggest that the current diagnostic practice of classifying such disorders as schizophrenia is reasonable. Rather, familial affective liability may play a role in increasing lower-level, but longer-lasting, depressed mood in schizophrenia.
The continuum viewpoint of Crow (
4) is another possible explanation for our findings. Crow hypothesizes that schizophrenia and affective disorders are genetically determined disorders on a continuum of severity, with the phenotypic expression dependent on variations in the form of the gene. The continuum viewpoint would account for the presence of affective disorders among the relatives as an indication of the presence of the psychosis-relevant allele, which for some offspring (including the probands) may have been modified across generations so that the resulting illness looks more like schizophrenia than affective disorder.
An important issue is whether some of the probands actually were experiencing a misdiagnosed affective disorder. The potential causes of misdiagnosis must be distinguished. One source might be the misapplication of the DSM-III-R criteria to these cases. This is a question of the interrater reliability of the diagnosticians who applied the criteria. To address this issue, the diagnosis of every patient entering the study was reviewed by a second diagnostician from the Diagnosis and Psychopathology Unit of the UCLA Clinical Research Center for Schizophrenia. Only data on patients whose diagnoses could be independently sustained were entered into these analyses.
A second potential source of misclassification might be that the DSM-III-R criteria were correctly and reliably applied but that the criteria are not fully valid for distinguishing between schizophrenia, schizoaffective disorder, and major affective disorder. This possibility cannot be ruled out and must be considered as a plausible alternative interpretation of the findings.
A third possibility is that these cross-sectional diagnoses were accurately made but that the subsequent course of illness of these patients might warrant a change to a diagnosis of an atypical affective disorder. This study differs from prior family studies of schizophrenia in that the probands were patients with recent onset of schizophrenia rather than chronic schizophrenic patients, and thus their ultimate illness course was not known at study entry. To examine this issue, the 15 patients who had a concurrent depressive syndrome at the inpatient hospitalization before study entry were rediagnosed 1 year after their index hospitalization. None of these 15 patients was diagnosed longitudinally as having DSM-III-R schizoaffective disorder or affective disorder. Thus, although additional longitudinal study of these recent-onset probands might reveal that the final form of illness in some cases meets criteria for an atypical affective disorder, the 1-year follow-up data provide no indication of misdiagnosis at project entry.