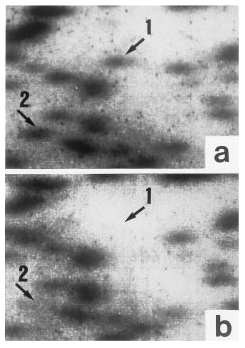
Restriction landmark genome scanning, developed for a high-speed survey of restriction sites throughout the genome, employs direct end-labeling of genomic DNA digested with a restriction enzyme and high-resolution, two-dimensional electrophoresis. As a spot's intensity accurately reflects the dose of a
NotI/
PstI restriction fragment, it is evident that twin A's genomic DNA contains two
NotI/
PstI fragments (spots 1 and 2), while the fragments are present in lower levels or are lacking in twin B. Since about three-fourths of monozygotic twins have in utero vascular connections (
3,
4), it is reasonable that twin B has a faint spot 2. The reason why no additional spots corresponding to spot 1 or 2 were visible anywhere in twin B's autoradiogram is most likely the limited resolution power of restriction landmark genome scanning, by which only DNA fragments that are 70–2,000 base pairs long can be detected. Differences derived from variable immunoresponse system genes are generally undetectable, because the number of such individual cells to be subjected to restriction landmark genome scanning is negligible.
Two alternative mechanisms by which the discrepant fragments in the twins appeared are possible. First, as we used the methylation-sensitive enzyme
NotI, the methylation status at one or more
NotI sites may be different in the two twins. Although previous studies did not support an association between the etiology of schizophrenia and genomic imprinting (
6), it is reasonable to assume the occurrence of epigenetic DNA modifications, such as methylation/demethylation, to explain the similar high rates of schizophrenia in the children of schizophrenic monozygotic twins and those of nonaffected co-twins (
1). Usually, methylation/demethylation is reset in the next generation. Second, in either twin A or B, a submicroscopic change of DNA, e.g., deletion, insertion, or translocation, may have occurred at one or more
NotI-flanking sites after (or simultaneous with) twinning. By either mechanism, since
NotI sites frequently exist in CpG islands near the promoters of genes (
7), the fragments have a high probability of reflecting such a mutation, leading to an alteration of the transcription level. In fact, from a
NotI-linking clone library, several genes have successfully been isolated (
8). Thus, it remains to be seen whether the genomic discordance observed in our monozygotic twins truly reflects different susceptibility to the disease. We are now trying to clone the discrepant DNA fragments with the gel-punching-out method (
9).
This new approach makes it possible to detect directly and rapidly different DNA fragments in monozygotic twins. Such a difference results from a postzygotic event. However, it seems meaningless to compare autoradiogram spots from restriction landmark genome scanning of unrelated individuals because of the presence of numerous restriction-fragment-length polymorphisms in the genome. In other words, DNA fragments with different sizes at the same locus on the genome cannot generally be identified in unrelated individuals. Moreover, since schizophrenia is a polygenic disorder and shows genetic heterogeneity, it would not be surprising if other monozygotic twins discordant for the disease had identical patterns in restriction landmark genome scanning.