Little information has been published about the use of selective serotonin reuptake inhibitors (SSRIs) during breast-feeding (
1). Altshuler et al. (
2) studied a nursling whose mother took 100 mg/day of sertraline and 125 mg/day of nortriptyline. At 3 and 7 weeks postpartum, the mother's sertraline levels were 48 and 47 ng/ml, respectively, and the infant's levels were less than 0.5 ng/ml on both occasions. Mammen et al. (
3) studied three infants aged 6–26 weeks. Sertraline and
N-desmethylsertraline were either not detectable or not quantifiable. Epperson et al. (
4) studied the serum levels of four infants whose lactating mothers took 50 or 100 mg/day of sertraline. All infant serum levels were less than 2.5 ng/ml of sertraline or 5 ng/ml of
N-desmethylsertraline. This group also evaluated the functional effects of very low levels of sertraline in infants by performing platelet serotonin analyses. Following sertra~line treatment, they observed the expected marked decline in maternal platelet serotonin levels but minimal change in the breast-fed infants.
Stowe et al. (
5) determined the serum concentrations of sertraline and
N-desmethylsertraline in 11 mother-infant pairs. Maternal doses ranged from 25 to 150 mg/day. Seven infants had nondetectable concentrations of sertraline, one had a nonquantifiable (less than 1 ng/ml) level, and the other three had levels of 3 ng/ml or less.
N-Desmethylsertraline was nondetectable in two infants, in three it was detectable at 1 ng/ml or less, and in six it was detectable at 10 ng/ml or less. No adverse effects were observed in the infants studied in any of the cited reports.
METHOD
Subjects. All of the mothers met DSM-IV criteria for major depression, and three had primary obsessive-compulsive disorder as well. None of the mothers or infants was taking other medications. We obtained informed consent for treatment with sertraline and for blood sampling. The healthy, full-term, fully breast-fed infants were 22 weeks old or younger at the time of serum sampling.
Sampling technique. We increased each mother's morning sertraline dose until her depression remitted or 200 mg/day was achieved. We collected steady-state samples after at least 14 days at a stable dose, except for the mother in case 1, whose samples were collected after 7 days. The half-lives of sertraline and
N-desmethylsertraline are 25 and 66 hours, respectively; therefore, the maternal levels should have reached steady-state in 5.2 and 13.8 days, respectively (
6). The serum levels of all nine women reached steady-state for sertraline, and all except the woman in case 1 reached steady-state for
N-desmethylsertraline.
Sample analyses. One of us (J.M.P.) analyzed the samples using high performance liquid chromatography with ultraviolet detection at 204 nm. The limit of reliable quantifiability was 2 ng/ml. The intraday coefficients of variation for sertraline for high and low control samples were 0.4% and 4.8%, respectively. The intraday coefficients of variation for N-desmethylsertraline for high and low control samples were 1.1% and 1.7%, respectively. The corresponding interday coefficients of variation for sertraline and N-desmethylsertraline ranged from 3.1% to 6.1%.
RESULTS
We detected sertraline or
N-desmethylsertraline at values between 0 and 2 ng/ml and reproducibly quantified levels greater than 2 ng/ml. Possible values are 0 (none detected), less than 2 ng/ml (detected but not quantifiable), and a numerical amount reproducibly quantified at greater than 2 ng/ml (
7).
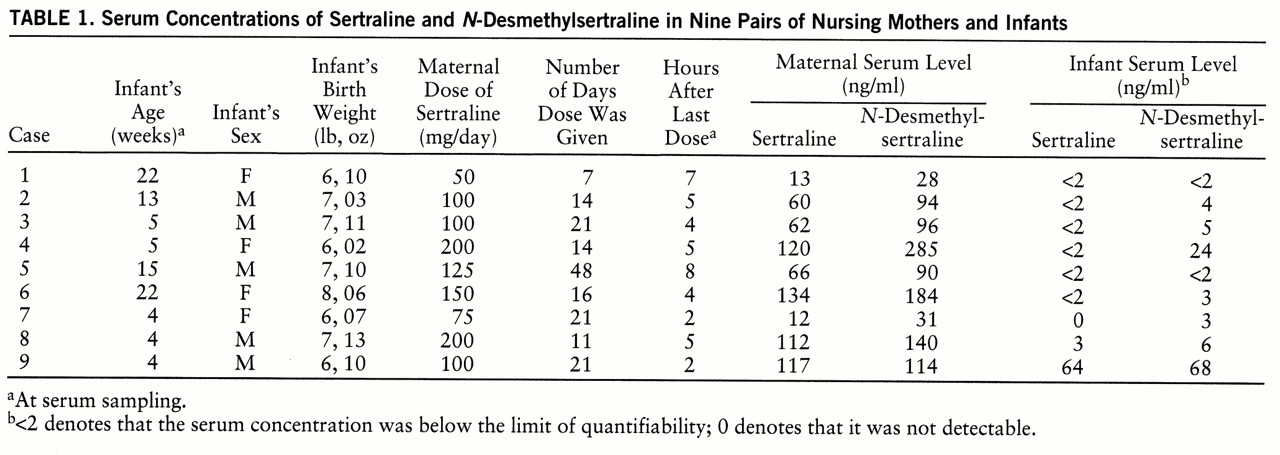
A summary of our results is given in
table 1. Maternal sertraline doses ranged from 50 mg/day to 200 mg/day. Sertraline levels were less than 2 mg/ml in seven of nine infants, and very low (3 ng/ml) in one.
N-Desmethylsertraline was not quantifiable in two infants and low (6 ng/ml or less) in five. We found a substantial level of
N-desmethylsertraline in infant 4 (24 ng/ml), despite no quantifiable serum sertraline. The mother of this infant stopped taking sertraline shortly after the blood sampling because her depression did not improve.
The infant in case 9 had serum levels of about half of the maternal values for both sertraline and N-desmethylsertraline. We review this case in the Discussion section. Neither parents nor pediatricians identified developmental or health problems in any of the infants throughout the time of serum sampling.
DISCUSSION
The sertraline levels of eight out of nine breast-fed infants whose mothers were taking sertraline were very low. Our study of nurslings revealed nonquantifiable levels (less than 2 ng/ml) in seven of nine infants and a very low level (3 ng/ml) in one. These data are consistent with those of Mammen et al. (
3), Epperson et al. (
4), and Stowe et al. (
5), who found sertraline levels below 2 ng/ml, 2.5 ng/ml, and 3 ng/ml or less, respectively, in nursing infants.
N-Desmethylsertraline was found in most of our nurslings. Trace amounts (2–6 ng/ml) were found in the sera of five of nine infants, and a substantial amount (24 ng/ml) was found in a sixth. The mothers of these infants typically had the highest serum levels in the series, particularly of
N-desmethylsertraline. The infant with the
N-desmethylsertraline level of 24 ng/ml had the mother with the highest sertraline dose and
N-desmethylsertraline serum level. Mammen et al. (
3) and Epperson et al. (
4) found infant serum levels of
N-desmethylsertraline below 2 and 5 ng/ml, respectively. However, the maximum dose used by these investigators was 100 mg/day of sertraline. Four of our mothers required doses greater than 100 mg/day for response, which accounts for our higher levels.
Our
N-desmethylsertraline findings are similar to those of Stowe et al. (
5), who found that nine of 11 infants had detectable
N-desmethylsertraline levels of 10 ng/ml or less. However,
N-desmethylsertraline does not contribute to antidepressant activity in behavioral models. In vitro data suggest that
N-desmethylsertraline has about one-eighth of the serotonin reuptake blockade potency of sertraline (
6).
= We evaluated our final case carefully because the infant's N-desmethylsertraline levels were unusual in that they were half the values for its mother. We checked the values twice. The infant was clinically thriving before blood was drawn. We considered several questions.
Could the infant develop this level with the dose delivered in breast milk? We can draw inferences about our case from data provided by Altshuler et al. (
2) for sertraline breast milk concentrations. Their case mother was the same age as our mother and took the same sertraline dose (100 mg/day). Our case mother had her serum level drawn 2 hours after receiving sertraline. According to Altshuler's data, the breast milk concentration 2 hours after dose administration is about half that at 5 hours, when peak breast milk concentration occurs. If we assume that our case mother's concentration at 2 hours after dose administration was half the maximal concentration achieved and double it, our infant received 2×117 ng/ml, or 234 ng/ml of sertraline in breast milk. If we assume that sertraline is fully unbound, which is unlikely, we can conclude that the baby received a maximum of 0.234 mg/day of sertraline (and probably
N-desmethylsertraline) if 1000 ml/day of breast milk was consumed. This very low oral dose makes the development of infant serum levels similar to those of directly treated mothers exceedingly unlikely.
Was there another source of sertraline? We wondered if this mother gave the infant sertraline. We were unable to contact her after the serum levels were drawn. Surreptitious administration of antidepressant to an infant has been described (
8).
Might there be another source of error? The serum is hand-pipetted and sent frozen for analysis. We cannot completely rule out processing contamination.
We believe that this infant's serum level was a spurious finding. The 18 published cases (
3–
5) plus our eight cases add up to 26 cases of low serum levels of sertraline and
N-desmethylsertraline in infants, which supports our contention that our final case is highly atypical.
In our opinion, sertraline use during breast-feeding is acceptable; however, a safe level of exposure to any agent is difficult to establish. Although these infants were thriving, chronic exposure to very low doses of antidepressants could affect infant neurodevelopment, which has not been systematically studied.