Pharmacological, neurobehavioral, and clinical research point to the possible implication of basal ganglia in schizophrenia. The basal ganglia represent target fields for dopaminergic tracts presumed to be important in the pathogenesis of schizophrenia. Basal ganglia play a critical role in higher cognitive functions such as attention, working memory, and goal-directed behavior (
1,
2). Lesions of basal ganglia may result in disturbances in thinking and behavior reminiscent of schizophrenia (
3). Involuntary movements were described in schizophrenia long before the era of neuroleptics (
4); unusual movements are also seen in preschizophrenic children long before illness onset (
5). Despite this compelling theoretical evidence, however, neuropathological studies of the basal ganglia have thus far revealed largely equivocal results (
3).
Magnetic resonance imaging (MRI) allows noninvasive in vivo examination of the structural abnormalities of the basal ganglia. Several MRI studies have appeared in the literature (
6–
23), and the results are conflicting (also see review by Shenton et al. [24]). Jernigan et al. (
6) first reported an increase in lenticular nuclei volume in schizophrenia. Since then, seven other studies have reported increased volume of one or other basal ganglia structures (
7–
11,
13,
14). Five studies have shown no differences in basal ganglia size (
12,
15,
16,
20,
23). Four studies have shown a reduction in caudate size in subpopulations of schizophrenic subjects characterized by tardive dyskinesia (
17,
18), negative symptoms (
19), or drug-induced parkinsonism (
21). These inconsistencies may be related to methodological issues, e.g., use of thick slices with interslice gaps, making it difficult to avoid partial volume effects (
8,
15,
20), or use of only area measures in some studies (
20,
21). Further, most of these studies involved previously treated schizophrenic patients, which suggests that the observed changes may be related to neuroleptic treatment.
Two studies have examined basal ganglia volume in early psychoses. DeLisi et al. (
8) observed a trend for reduced caudate volume in first-episode schizophrenic patients by comparison with chronic schizophrenic and healthy comparison subjects. Chakos et al. (
12) did not find differences between first-episode schizophrenic and healthy comparison subjects. In both of those studies, most patients had received neuroleptic medications before the baseline MRI scan. Few studies have examined basal ganglia volumes in neuroleptic-naive schizophrenic patients. Such studies can help clarify whether basal ganglia abnormalities are secondary to neurolep~tic treatment or whether they are primary to the illness.
In this study, we conducted MRI morphometric studies of the basal ganglia in newly diagnosed neuroleptic-naive schizophrenic and matched healthy comparison subjects. We also examined a group of neuroleptic-naive non~schizophrenic psychotic patients in order to address the issues of diagnostic specificity. Follow-up neuroimaging data have been previously published from a subset of these data (
22). In this report, we provide new data on basal ganglia volume measures at baseline. We have focused on the caudate and putamen nuclei, since they are the most easily and reliably measured among basal ganglia nuclei. We were unable to obtain satisfactory interrater reliability with the globus pallidus and therefore did not measure this structure.
METHOD
Sixteen first-episode neuroleptic-naive schizophrenic patients (schizophrenia, N=13; and schizoaffective disorder, N=3), nine nonschizophrenic psychotic patients (bipolar disorder with psychotic features, N=3; major depression with psychotic features, N=3; and psychotic disorders not otherwise specified, N=3), and 17 normal comparison subjects were studied. All subjects provided fully informed written consent. The patients were diagnosed by DSM-III-R criteria at consensus meetings incorporating all clinical, follow-up, and Structured Clinical Interview for DSM-III-R (SCID) (
25) data. Normal comparison subjects were recruited from neighborhoods in which the patients resided, were assessed with the SCID (nonpatient version), and were matched, as a group, with the schizophrenic patients for age, sex, race, and parental socioeconomic status (
26). Exclusion criteria included significant medical illness, substance abuse or dependence, prior neuroleptic treatment, and IQ lower than 70. Illness durations were computed from the onset of prodromal symptoms to entry into the study. In order to obtain the onset dating as accurately as possible, we timed the dates of onset of the first identifiable psychotic and prodromal symptoms on the basis of a best-estimate approach using data gathered from multiple sources, including the medical record, direct patient interview, and, when possible, a family interview. Dating of the onset of the first prodromal or psychotic symptom was estimated to the closest month (estimated range of error=±1 month), when possible. The duration for the schizophrenic group (N=15) was 301.35 weeks (SD=269.14, range=8–823). For one patient, it was difficult to determine the age at onset. The illness duration in the nonschizophrenic psychotic group was 155.06 weeks (SD=159.04, range=6–463).
MRI scans were conducted with a 1.5-Tesla General Electric Signa system (General Electric Medical Systems, Milwaukee) at the University of Pittsburgh Medical Center. A spin echo sagittal scout scan and axial scans (slice thickness=2.8 mm; no interslice gap; three-dimensional spoiled gradient recalled acquisition pulse sequence; TR=40 msec; TE=20 msec) were acquired. They were identified by scan number alone to retain blindness and were analyzed on a MacIntosh workstation with IMAGE software (version 1.55) (
27) by trained raters, through use of a semiautomated segmentation algorithm (
28). The right and left caudate nuclei were measured by using a manual tracing technique (
22,
29). Intracranial volume was calculated by summing up areas of successive axial slices including gray matter, white matter, and CSF and multiplying by slice thickness. Mean number of slices was as follows: right caudate, 11.5; left caudate, 11.5; right putamen, 7.1; and left putamen, 7.2. Intrarater reliabilities (N=10) ranged from 0.90 to 0.97 for caudate and from 0.92 to 0.94 for putamen. Interrater reliabilities in our laboratory (W. Bagwell, E. Dick, M. Zeigler, and T. Kisler) (N=10; intraclass r) were high for caudate (r=0.94), putamen (r=0.97), and intracranial volume (r=0.99) but were poor for globus pallidus (r=0.26).
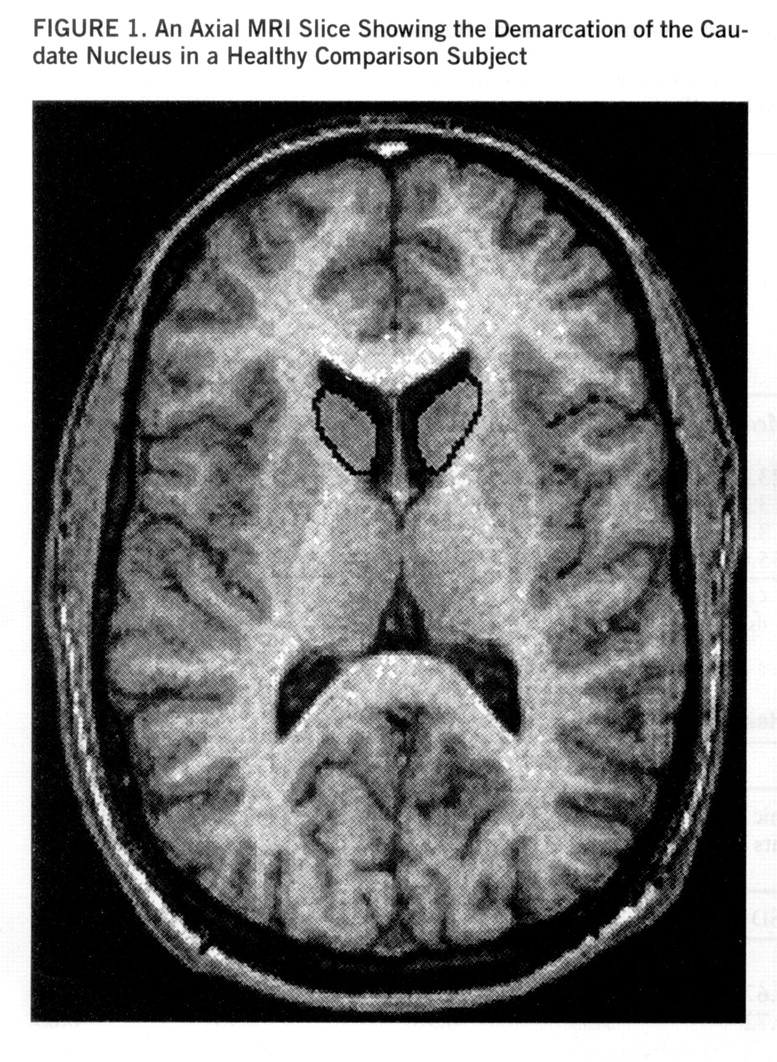
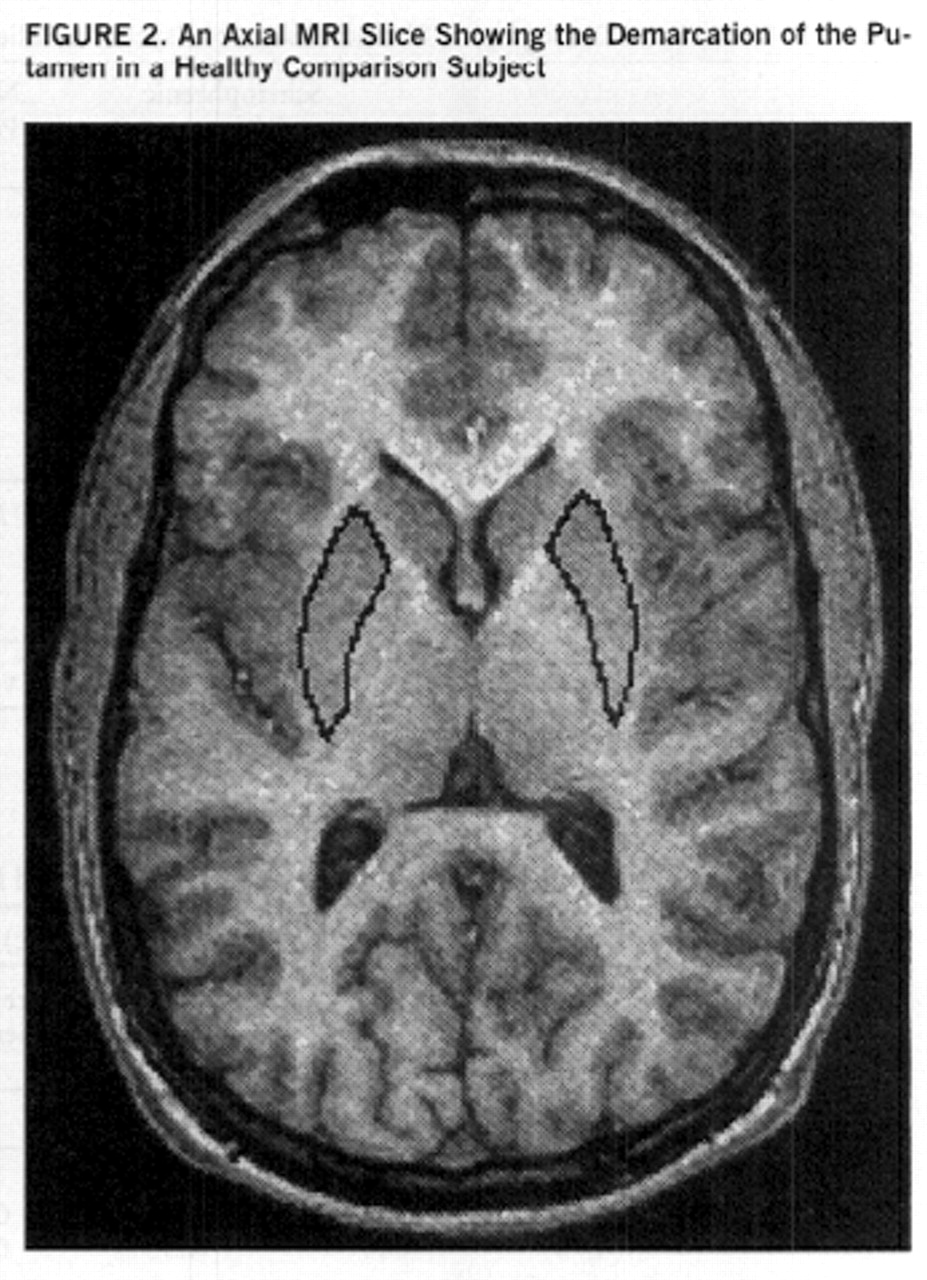
Separate measurements were obtained for the left and right caudate nuclei through use of a manual tracing technique. The head and body of the caudate were measured, whereas the tail was excluded. The caudate nucleus is delineated medially and superiorly by the lateral ventricle and elsewhere by frontal white matter; it is separated from the putamen by the white matter tracts of the anterior limb of the internal capsule. We included the nucleus accumbens, which merges inferiorly with the caudate; it is hard to reliably demarcate this structure from the caudate nucleus. All regions were measured in the axial plane.
Separate measurements were obtained for the left and right putamen by using manual tracing. The putamen is delineated medially by the globus pallidus and laterally and inferiorly by white matter tracts of the external capsule. The caudate and the putamen are connected inferiorly by gray matter bridges; because of the partial volume effects, these are excluded from the measurements. Measurements of the putamen were performed in the axial plane (figures
1 and
2).
We conducted analysis of covariance (ANCOVA), with intracranial volume as a covariate, and Newman-Keuls post hoc tests to examine significant differences between diagnostic groups; we used Pearson's correlations and partial correlations to examine the relation among age, illness duration, and caudate and putamen volume. For dichotomous variables such as sex and handedness, we used the Kruskal-Wallis analysis of variance (ANOVA). All significance values reported are two-tailed.
RESULTS
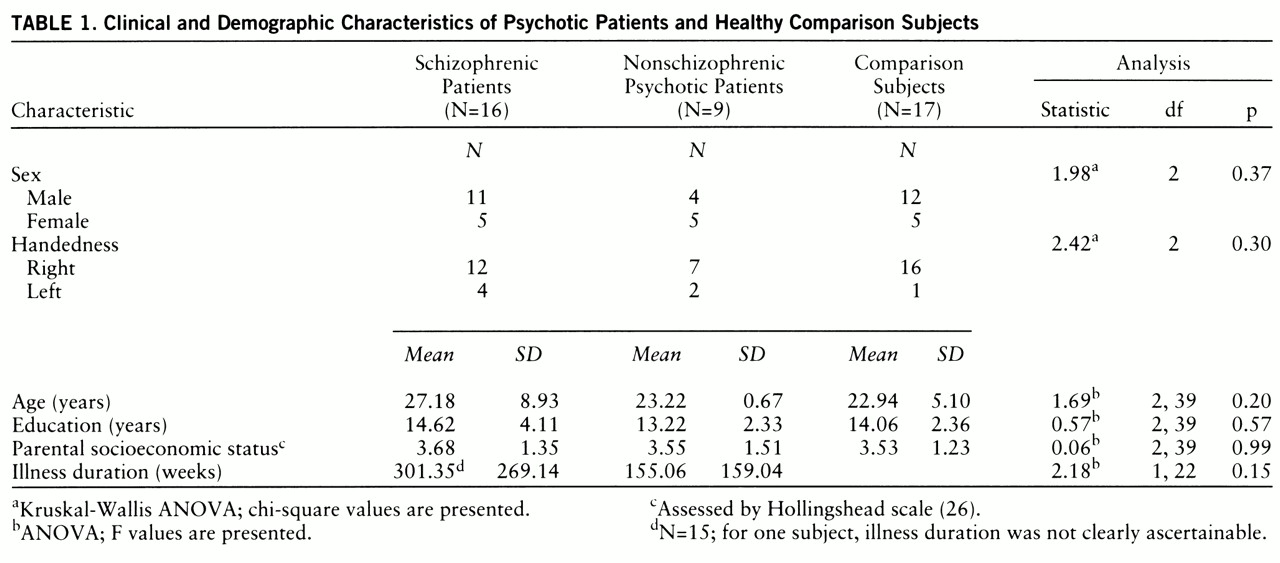
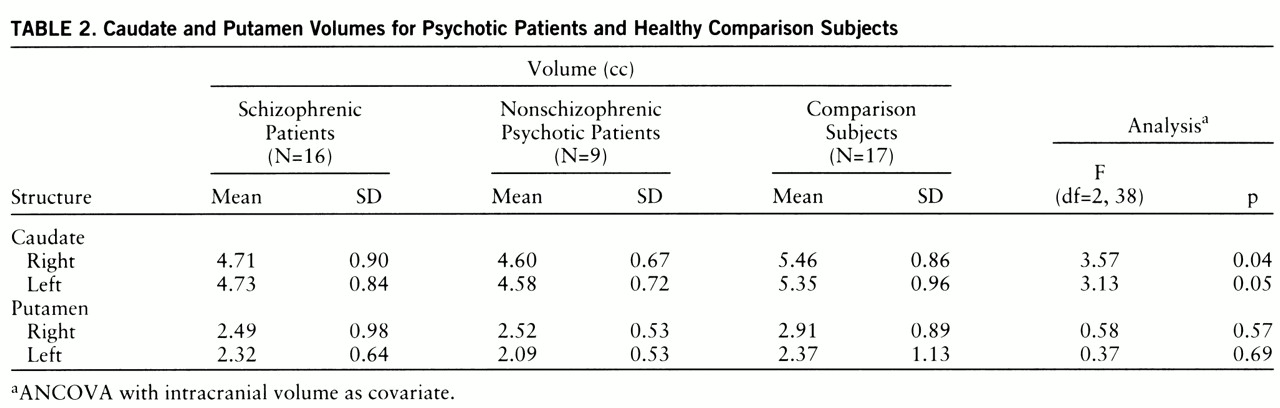
There were no significant differences among the three groups in age, sex, education, handedness, or parental socioeconomic status (
table 1). Age did not correlate with caudate and putamen volumes in any of the diagnostic groups. Intracranial volume was not significantly different among the groups (schizophrenic patients: mean=1476.99 cc, SD=185.33; nonschizophrenic psychotic patients: mean=1528.93 cc, SD=17.68; comparison subjects: mean=1576.57 cc, SD=172.97) (F=1.41, df=2,39, p=0.26). ANCOVA, with intracranial volume as a covariate, revealed significant intergroup differences in both left and right and in total caudate volume (
table 2). Post hoc tests revealed that both patient groups had smaller right and left caudate volumes than the comparison subjects. Illness duration (after intracranial volume was partialled out) was unrelated to caudate or putamen in both the schizophrenic and the nonschizophrenic psychotic groups (all partial r values <0.5). No significant differences were seen between the subject groups for right or left putamen.
DISCUSSION
Significant reductions (about 14%) were seen in caudate volumes in neuroleptic-naive schizophrenic patients. These findings may be diagnostically nonspecific, since similar volume reductions were also seen in nonschizophrenic psychotic patients; reduced caudate volume has also been described in depression (
30). In a prospective study of a subset of these first-episode psychotic patients, we (
22) and others (
10) have found that caudate volumes increase following treatment with neuroleptics. Previous observations of increased caudate volume in schizophrenia may therefore reflect neuroleptic effects. Thus, caudate volume reduction in the neuroleptic-naive patients may reflect primary pathophysiology of schizophrenia and may account for some of the cognitive and psychomotor abnormalities in this illness.
Our observations of caudate, but not putamen, volume reductions in neuroleptic-naive psychotic patients deserve comment. The putamen mainly receives projections from cortical regions involved in motor control (
31,
32). On the other hand, recent studies in primates have shown that the caudate nuclei are clearly activated during working memory-related tasks (
2). Thus, this structure may be part of a distributed neuronal network subserving functions associated with the dorsolateral prefrontal cortex, implicated in the pathogenesis of schizophrenia. Our observations are also consistent with functional neuroimaging research. Significantly reduced basal ganglia metabolism has been observed in unmedicated schizophrenic patients through use of positron emission tomography (
33–
35) and single photon emission tomography (
36). On the other hand, treated schizophrenic patients had higher blood flow in basal ganglia (
37,
38). Indeed, one study showed decreased caudate metabolism at baseline that reversed following institution of neuroleptic treatment (
34).
The lack of association between caudate volume and illness duration should be viewed with caution, since studies of patients with longer duration of illness may reveal the effects of chronicity. Nevertheless, this finding and the observation of caudate volume reduction early in the illness provide some indirect support for neurodevelopmentally mediated pathology in schizophrenia (
39) and possibly in affective disorder (
40). An exaggeration of periadolescent synaptic pruning, perhaps in glutamatergic corticosubcortical neurons, may be involved (
41). Reduced activity in these cortico~striatal neurons, by diminishing trophic effects on the striatum, could conceivably lead to reduced synaptic neuropil, and thereby reduced size; this view is consistent with a recent observation of reduced striatal dendritic spine size in postmortem brains of schizophrenic patients (
42).
Many brain regions have been implicated in the neuroanatomy of schizophrenia. Our findings suggest that the relevant brain circuits underlying the pathophysiology of schizophrenia are likely to include the caudate nuclei; however, several other regions and abnormal patterns of interregional cross-talk may be involved in this disorder. Our findings must be considered preliminary in view of the relatively small study group size. Further studies of basal ganglia structure and metabolism, through use of state-of-the-art neuroimaging techniques, are clearly warranted.