Insomnia is a frequent complaint among patients with major depressive disorder. Newer-generation antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs), seem to be better tolerated by some patients but generally have little effect on sleep. This may be a limitation for patients with marked sleep complaints and often requires the addition of benzodiazepines. The use of benzodiazepines raises concerns regarding tolerance, habituation, and dependence.
Melatonin, a hormone secreted by the pineal gland, reportedly plays a critical role in the regulation of the day-night cycle. Its hypnotic effects have been demonstrated in patients with primary sleep disorders (
1), elderly insomniacs (
2), aircrews with jet lag (
3) and normal volunteers (
4). In all these studies, melatonin was found to be effective in inducing sleep and to have no notable side effects. Previous studies addressing the use of melatonin in psychiatric disorders have been mostly limited to seasonal affective disorder. No significant effects of melatonin were described in those studies (
5).
To test whether melatonin would demonstrate sleep-inducing effects in patients with major depressive disorder, we treated 19 patients with major depressive disorder in a double-blind, placebo-controlled fashion. A slow-release preparation of melatonin was used. Following its administration, slow-release melatonin is released in a profile that simulates that of the endogenous melatonin profile (U.S. patent 5,498,423). Slow-release melatonin has been reported to have better all-night effects (
6). Regular melatonin is available in a variety of doses, most commonly 1 to 5 mg. These doses result in serum melatonin concentrations that are 10 to 100 times higher than the usual nighttime peak within 1 hour after ingestion, followed by a decline to baseline values within 4–8 hours (
7).
In addition to slow-release melatonin, all patients received standard fluoxetine treatment. We chose fluoxetine as a representative SSRI, which often requires the use of hypnotic medication during the initial stages of treatment.
The effects of slow-release melatonin were assessed during the first 4 weeks of fluoxetine treatment. To the best of our knowledge, this is the first report on the use of melatonin in patients with major depressive disorder.
METHOD
The 24 patients included in the study were 22–65 years of age and were recruited from the psychiatric outpatient clinic at the Sheba Medical Center in Israel. All patients were diagnosed as suffering from major depressive disorder according to DSM-IV on the basis of a clinical interview and a checklist of symptoms. The minimal 21-item Hamilton Depression Rating Scale (
8) score on recruitment was 16 points. Patients with psychotic depression, bipolar disorder, schizoaffective disorder, or primary sleep disorders were excluded during the initial interview. Patients had no other substantial physical illnesses and were not taking any other hypnotic medications, such as antihistamines. The research protocol was approved by the human use committee of our institution and by the Ministry of Health. After complete description of the study to the subjects, written informed consent was obtained.
All patients received 20 mg of fluoxetine as the sole antidepressant medication. Slow-release melatonin was supplied by Neurim Pharmaceuticals, Israel. Slow-release melatonin or placebo were given in 5-mg capsules; this dose could be increased up to 10 mg in increments of 2.5 mg. Dose of slow-release melatonin could be increased as of week 2 of the study if the patient's Pittsburgh Sleep Quality Index score (
9) did not improve by at least 20%. The capsules were taken at 9:00 p.m. Patients were randomly assigned to either treatment in a double-blind fashion. All ratings were performed by the principal investigator (O.T.D.), who was blind to the study conditions. Ratings were performed at baseline and after 2, 3, and 4 weeks; the 21-item Hamilton depression scale (
8), the Brief Psychiatric Rating Scale (BPRS) (
10), and the Pittsburgh Sleep Quality Index (
9) were used. The Pittsburgh Sleep Quality Index is a self-rated questionnaire designed specifically to measure sleep quality in clinical populations; it yields seven component scores and a global score. The seven components are subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Statistical analysis included chi-square tests for categorical data such as demographic variables and t tests or analysis of variance (ANOVA) with repeated measures for continuous data.
RESULTS
Nineteen of the 24 patients originally included in the study completed the study. Five patients dropped out; one because of failure to improve and four because of side effects of fluoxetine. In addition to fluoxetine, 10 patients received melatonin and nine received placebo. No statistically significant differences were found between the groups receiving melatonin and placebo regarding sex, age, marital status, level of education, number of previous depressive episodes, or length of current episode.
Side effects such as diarrhea (N=2), constipation (N=5), epigastric fullness (N=1), and headaches (N=3) were attributed to treatment with fluoxetine and were not significantly different between patients treated with slow-release melatonin and those given placebo. Daytime somnolence (N=3) attributed to slow-release melatonin improved when medication was taken at the recommended hour (9:00 p.m.).
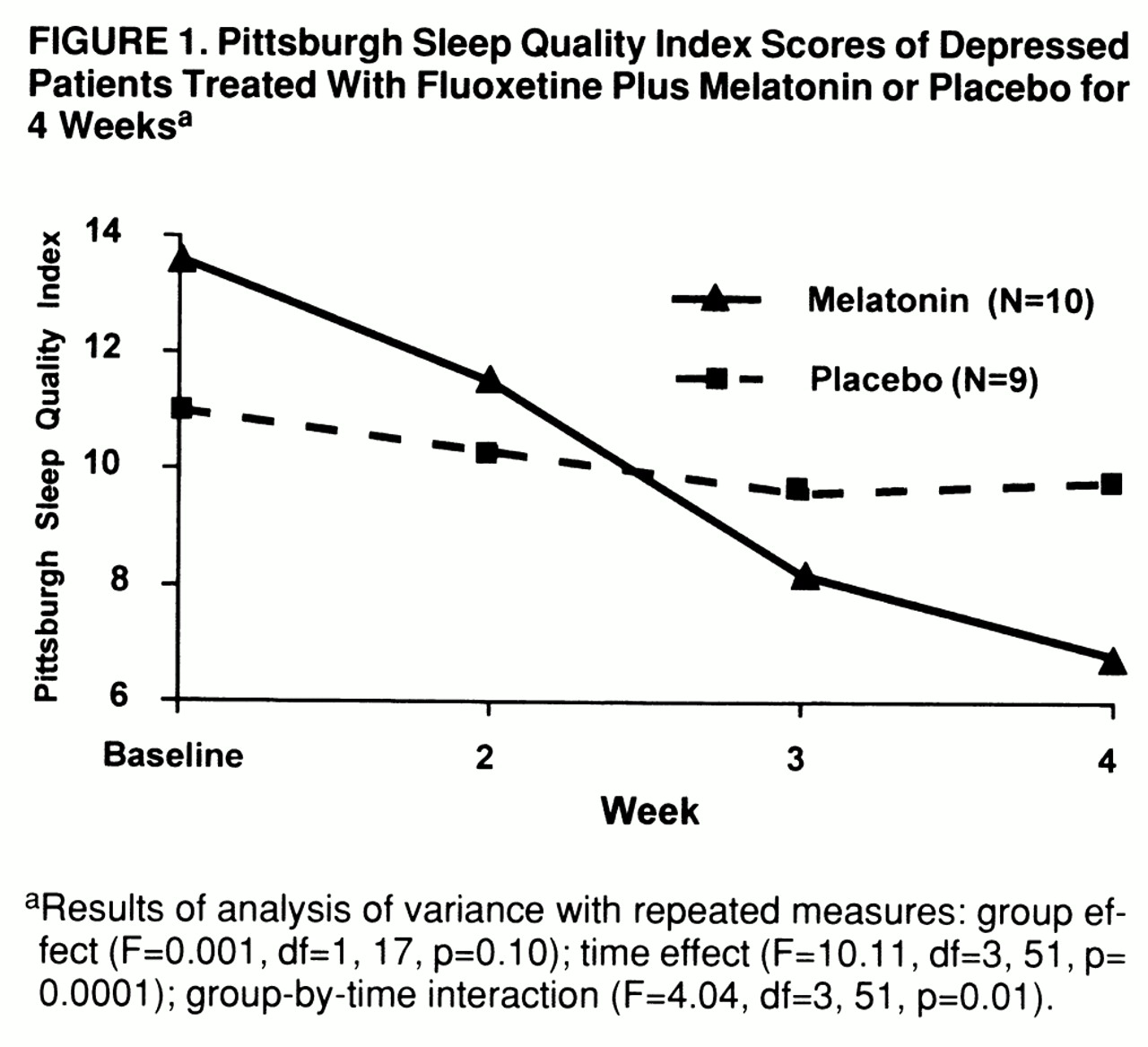
Baseline scores on the BPRS and the Hamilton depression scale were not significantly different between the two groups of patients. In addition, at week 4, no significant differences were noted between the two groups of patients in Hamilton depression scale or BPRS scores, regardless of whether sleep-related questions were included or not. However, patients treated with slow-release melatonin showed statistically significant improvement in sleep variables on the Pittsburgh Sleep Quality Index than did patients treated with placebo (F=4.04, df=3,51, p=0.01, repeated measures ANOVA) (
figure 1). Slow-release melatonin's effect was most evident on subjective sleep quality (t=2.24, df=17, p=0.38, unpaired t test), and sleep length (t=2.55, df=17, p=0.03, unpaired t test).
DISCUSSION
In this study of patients with major depressive disorder, those who received slow-release melatonin along with standard antidepressant treatment with fluoxetine reported better sleep than those who did not receive slow-release melatonin. Slow-release melatonin appears to be an effective and safe treatment for sleep disturbances observed in major depressive disorder, particularly those of sleep continuity measures. Slow-release melatonin did not accelerate the onset of the antidepressant actions of fluoxetine, however. Although studies including larger samples are needed before definite conclusions can be drawn, the addition of slow-release melatonin to standard treatment with SSRIs appears to avoid the need for additional sleep medications.