Treatment with conventional dopamine receptor-blocking antipsychotics—although effective in the treatment of positive symptoms (e.g., hallucinations)—has relatively limited effects on negative symptoms (e.g., social withdrawal, blunted emotional responses) in schizophrenia. An alternative strategy consistent with the hypoglutamatergic hypothesis of schizophrenia
(1–
3) involves the administration of compounds that stimulate
N-methyl-
d-aspartic acid (NMDA)-mediated activity through the strychnine-insensitive glycine site. In a subgroup of chronic schizophrenic subjects, the addition of glycine to conventional neuroleptic treatments decreased negative symptoms
(4–
9) without worsening positive symptoms
(10).
d-Cycloserine, a partial agonist at the glycine site, may improve negative symptoms when added to conventional neuroleptics
(11); it does not appear to be effective at higher doses
(11,
12) and may worsen negative symptoms in patients maintained on clozapine
(12).
In studies of adjunctive glycine, the majority of patients were maintained on a variety of neuroleptics
(4–
9). The focus of the current study was to determine whether glycine augments the therapeutic efficacy of the atypical neuroleptic clozapine in patients with treatment-resistant schizophrenia.
METHOD
Twenty-four chronic schizophrenic patients (21 male and three female) who met the DSM-III-R criteria for schizophrenia and were free of other axis I diagnoses or clinically significant concurrent medical illnesses were enrolled in this 12-week study. They were chronically hospitalized (mean=12.4 years, SD=7.2) at Metropolitan State Hospital, Norwalk, Calif., and were maintained on optimal doses of clozapine (range=400–1200 mg/day) for several months before the start of the trial.
Although improvement was observed with clozapine, these patients remained symptomatic (mean BPRS score=43.2, SD=12.2) and required continual hospitalization. The patients were randomly assigned to a glycine group (10 men and two women) or a placebo group (11 men and one woman). There was no significant difference between the groups in age (mean=35.3 years, SD=5.26, and mean=34.4 years, SD=4.75, respectively) or clozapine dose (mean=589 mg/day, SD=172, and mean=635 mg/day, SD=232, respectively). After a complete description of the study to the subjects, written informed consent was obtained.
Patients in the glycine group were administered a solution of glycine (10 g) dissolved in 1 oz of water three times a day after meals. The placebo group received 1 oz of a similar-tasting solution three times a day after meals. Physician raters, nurses, and the patients were kept blind to the procedure. Raters received biweekly training sessions to maintain adequate interrater reliability for all scales. Standardized rating scales (the Brief Psychiatric Rating Scale [BPRS], the Scale for the Assessment of Negative Symptoms [SANS]
[13], the Simpson-Angus movement scale
[14], and the SAFTEE adverse symptoms checklist
[15]) were administered at baseline and at weeks 2, 4, 6, 8, and 12 during the trial. Clozapine levels and laboratory studies (e.g., SMA-17 and CBC) were evaluated at baseline and at weeks 6 and 12.
RESULTS
Because of an inability to participate or lack of cooperation, three patients (one female and two male) from the glycine group and two patients (one female and one male) from the placebo group were discontinued from the study prior to week 8. Data analysis was done on the remaining 19 patients (glycine group: eight men and one woman; placebo group: 10 men). One-way analysis of variance (ANOVA) was performed on the baseline data. There were no between-group differences in baseline total BPRS score (glycine group: mean=40.4, SD=12.2; placebo group: mean=45.9, SD=9.9; F=1.05, df=1, 16, p=0.32), in BPRS positive symptoms score (glycine group: mean=13.3, SD=5.7; placebo group: mean=17.4, SD=5.0; F=2.49, df=1, 16, p=0.13), or in SANS score (glycine group: mean=13.7, SD=6.1; placebo group: mean=13.7, SD=3.6; F=0, df=1, 16, p=0.99).
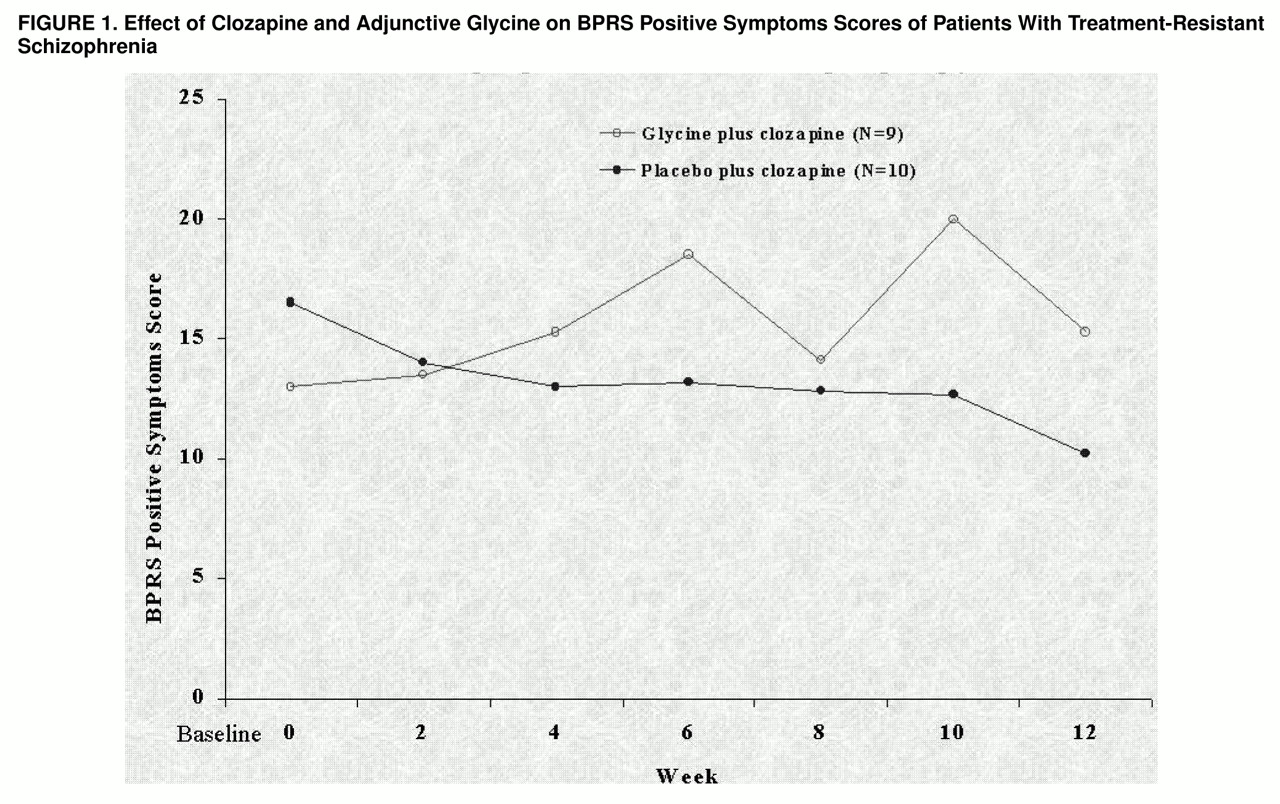
Repeated measures ANOVA with baseline values as covariates across the 12-week study showed significant improvement in BPRS positive symptoms in the patients treated with clozapine alone compared to the patients treated with clozapine and glycine (F=5.75, df=1, 16, p=0.03) (
figure 1). There were no significant treatment effects on negative symptoms as measured with the SANS (F=0.27, df=1, 16, p=0.61) or the BPRS (F=0.45, df=1, 16, p=0.51) or on overall psychopathology as measured by the total BPRS score (F=0.99, df=1, 16, p=0.33). The adjunctive glycine was well-tolerated throughout the double-blind study. No adverse changes in hematological values or serum chemistry were observed. In addition, adjunctive glycine did not significantly affect clozapine blood levels (posttest level for the glycine group: mean=511.4, SD=159.1; for the placebo group: mean=642.0, SD=255.8; F=1.32, df=1, 16, p=0.27, ANOVA).
DISCUSSION
To our knowledge, this is the first study to systematically evaluate the efficacy of glycine as an adjunct to clozapine, an atypical antipsychotic. Results from this double-blind study show that adjunctive glycine does not augment the antipsychotic effects of clozapine. These findings are in contrast to our findings in open
(4) and double-blind
(7) studies as well as data from other laboratories
(6,
8), all of which used conventional neuroleptics. Differential responses to glycinergic compounds as adjuncts to treatment with conventional and atypical neuroleptics have been reported elsewhere. Although adjunctive
d-cycloserine, a glycinergic compound, improves negative symptoms in patients maintained on conventional neuroleptics
(11,
12), it increases negative symptoms in patients maintained on the atypical antipsychotic clozapine
(16).
In the present study, patients administered glycine failed to show the improvement in positive symptoms that occurred in clozapine-treated patients who did not receive glycine. It has been suggested by Goff et al.
(16) that the lack of therapeutic efficacy of clozapine and adjunctive glycinergic drugs may be due to effects of clozapine on NMDA-mediated systems. We did observe a 5% greater improvement in negative symptoms in patients treated with adjunctive glycine compared with those who received placebo, but it did not reach statistical significance. This observation must be cautiously interpreted, as we had less than 10% power to detect such a small difference
(17).
In summary, the data suggest that the addition of high-dose glycine to clozapine therapy may not be effective in patients with treatment-resistant schizophrenia, and, if anything, glycine may decrease the efficacy of clozapine (as seen by the lack of improvement in positive symptoms in the glycine group). These findings provide interesting data for the further study of glycine and its differential interactions with typical and atypical antipsychotics in chronic schizophrenia.