Childhood sexual abuse is extremely common in our society; 16% of women are the victim of rape, attempted rape, or molestation at some time before their 18th birthday
(1). Childhood sexual abuse is the most common cause of posttraumatic stress disorder (PTSD), which affects 10% of individuals in this country according to recent nationwide surveys
(2). Women with childhood sexual abuse often are troubled by recurrent, intrusive memories of their childhood abuse experiences; increased fearfulness and startle reactions; hypervigilance; difficulty with concentration; and problems with emotional numbing that affect their interpersonal relationships. In spite of the high prevalence of childhood sexual abuse and PTSD in our society, little is known about the long-term effects of abuse on brain function.
Studies in animals have mapped out several interconnected brain regions that are involved in emotion and the stress response. These brain areas, which appear to have functional significance for PTSD and the response to threat
(3,
4), share in common the fact that they mediate different aspects of memory and visuospatial processing. Medial prefrontal cortex consists of several related areas, including orbitofrontal cortex, anterior cingulate (area 25 [subcallosal gyrus], and area 32), and anterior prefrontal cortex (area 9). The medial prefrontal dopaminergic system is one of the most sensitive areas in the brain to even mild stressors
(5). Lesions in this area result in a failure to mount the peripheral cortisol and sympathetic response to stress
(6–
8). This area also has important inhibitory inputs to the amygdala that mediate extinction to fear response. Animals with lesions of the medial prefrontal cortex are unable to extinguish fear responses after trials of fear conditioning
(9). Human subjects with lesions of the prefrontal cortex show dysfunction of normal emotions and an inability to relate in social situations that require correct interpretation of the emotional expressions of others
(10). These findings suggest that dysfunction of medial prefrontal cortex may play a role in pathological emotions that sometimes follow exposure to extreme stressors such as childhood sexual abuse.
Other brain areas that are interconnected with medial prefrontal cortex play an important role in the stress response. The amygdala plays a central role in conditioned fear responses
(7,
11). The declarative memory functions of the hippocampus are important in accurately identifying the signal of potential threat during stress situations. The hippocampus is also involved in fear responses to the context of a stressful situation
(12,
13). Stress results in damage to hippocampal neurons with associated deficits in memory
(14,
15). Posterior cingulate, parietal and motor cortex, and cerebellum are functionally related to anterolateral prefrontal cortex (superior and middle frontal gyri)
(16), mediating visuospatial processing that is critical to survival in life-threatening situations
(6,
8). The excessive vigilance seen in PTSD may be associated with increased demands on brain areas involved in visuospatial aspects of memory function and planning of response to potentially threatening stimuli.
Neuroimaging studies have demonstrated abnormalities in brain areas involved in memory in patients with PTSD
(17). To date, most of these studies involved male Vietnam combat veterans with PTSD. Patients with combat-related PTSD had smaller hippocampal volume than comparison subjects, on the basis of magnetic resonance imaging
(18,
19). Decreased metabolism measured with positron emission tomography (PET) was found at baseline in temporal and prefrontal cortex in patients with combat-related PTSD
(20) and in parietal cortex in patients with PTSD and comorbid substance dependence
(21). Provocation of PTSD symptoms with the pharmacologic agent yohimbine (which stimulates brain norepinephrine release) resulted in a relative failure of orbitofrontal cortex activation in PTSD patients relative to comparison subjects, as well as in differential functional responses to challenge in temporal, parietal and prefrontal cortex. These findings were consistent with increased noradrenergic responsiveness to yohimbine in patients with PTSD
(20).
Exposure of patients with PTSD to traumatic scripts resulted in increased blood flow in limbic regions (right insula/amygdala region, orbitofrontal cortex, and anterior cingulate) and decreased blood flow in middle temporal and left inferior frontal cortex, as measured with PET
(22). A meta-analysis of a variety of anxiety induction tasks in patients with different anxiety disorders did not find activation in anterior cingulate
(23), unlike studies of normal subjects, which have consistently reported anterior cingulate activation with emotional and anxiety arousing tasks. Exposure to trauma-related mental imagery in patients with combat-related PTSD resulted in increased blood flow in right amygdala and anterior cingulate and decreased blood flow in middle temporal and left inferior frontal cortex
(24). We used PET to measure blood flow during exposure to combat-related slides and sounds in Vietnam combat veterans with and without PTSD and found decreased blood flow in medial prefrontal cortex in subcallosal gyrus (area 25), with a failure of activation in anterior cingulate (area 32), in veterans with PTSD relative to veterans without PTSD. PTSD patients also had relative increases in blood flow in posterior cingulate, motor cortex, inferior parietal cortex, and lingual gyrus (posterior parahippocampus) and decreased blood flow in middle temporal gyrus
(25). Studies of subjects with abuse-related PTSD showed smaller hippocampal volume than in healthy comparison subjects
(26,
27). The method of reading scripts of traumatic events that are specific to a person’s experience has been used to reliably provoke PTSD symptoms and physiological markers such as galvanic skin response in patients with PTSD
(28–
33).
The purpose of the present study was to use PET in the examination of neural correlates of memories of childhood sexual abuse. Women with a history of childhood sexual abuse listened to scripts of childhood sexual abuse events, and brain blood flow was compared for women with and without PTSD. On the basis of the findings reviewed earlier in this article, we hypothesized that exposure of women with and without PTSD to scripts of childhood sexual abuse would result in group differences in activation patterns in medial prefrontal cortex (subcallosal gyrus and other parts of anterior cingulate), posterior cingulate, motor and parietal cortex, and hippocampus and adjacent cortex.
METHOD
Twenty-two women with a history of severe childhood sexual abuse (rape, genital fondling, or attempted rape before the age of 18) participated in the study. Subjects included women with (N=10) and without (N=12) PTSD related to childhood sexual abuse. All subjects were recruited through newspaper advertisement. Diagnosis of PTSD was established with the Structured Clinical Interview for DSM-IV (SCID)
(34). All subjects gave written informed consent for participation; were free of major medical illness on the basis of history and physical examination, laboratory testing, and ECG; were not actively abusing substances or alcohol (in the past 6 months); and were free of all medications for at least 4 weeks before the study. Subjects did not discontinue medication for the purposes of participating in the study. Subjects with a serious medical or neurological illness, organic mental disorders or comorbid psychotic disorders, retained metal, a history of head trauma, loss of consciousness, cerebral infectious disease, or dyslexia were excluded. There were no differences in age between the sexually abused women with (mean=35 years, SD=6) and without (mean=32 years, SD=8) PTSD.
History of childhood abuse was assessed with the Early Trauma Inventory. The Early Trauma Inventory is a 56-item clinician-administered interview that assesses physical, emotional, and sexual abuse, as well as general traumatic events. The Early Trauma Inventory has been demonstrated to be reliable and valid in the assessment of childhood trauma (J.D. Bremner et al., unpublished data). As noted earlier, all women had a history of severe sexual abuse, defined as rape, attempted rape, or molestation, before their 18th birthday. However, women with PTSD still had greater severity of sexual abuse, as measured by the Early Trauma Inventory Sexual Abuse Severity Index (for women with PTSD, mean score=948, SD=711; for women without PTSD, mean=71, SD=136). On the basis of the assessment of childhood abuse with the Early Trauma Inventory, the subject, with the assistance of the interviewer, prepared a personalized script of a severe childhood sexual abuse event; the methods used have been previously described
(28–
33). Two scripts were prepared, each 1 minute in length, that described either two aspects of a single traumatic event that the person experienced or two different events. These scripts were later read aloud to the subject during the scanning session.
Eight (80%) of 10 patients with PTSD met criteria for a lifetime history of major depression and two (20%) for current major depression, according to the SCID interview. One patient (10%) met criteria for a past history of dysthymia. Two of 10 patients fulfilled criteria for lifetime history of panic disorder with agoraphobia and two (20%) for current panic disorder with agoraphobia; one met criteria for lifetime history of panic disorder without agoraphobia. Three patients (30%) fulfilled criteria for a lifetime history of alcohol dependence, one for lifetime polysubstance dependence, one for lifetime marijuana dependence, and one for lifetime cocaine dependence. Among the comparison group of sexually abused women without PTSD, three (25%) of 12 fulfilled criteria for a lifetime history of major depression and none for current major depression, according to the SCID interview. One (8%) of 12 patients fulfilled criteria for current panic disorder with agoraphobia. One of 12 fulfilled criteria for lifetime obsessive-compulsive disorder, one for lifetime generalized anxiety disorder, and one (8%) for lifetime anorexia. One patient met criteria for lifetime marijuana dependence, one patient for lifetime marijuana abuse, and one for lifetime cocaine dependence.
Each subject underwent four scans on a single day. The subject was placed in the scanner with her head in a holder to minimize motion and positioned with the canthomeatal line parallel to an external laser light. An intravenous line was inserted for administration of [
15O]H
2O. Following positioning within the camera gantry, a transmission scan of the head was obtained by using an external
67Ga/
68Ge rod source, in order to correct emission data for attenuation due to overlying bone and soft tissue. Ratings on the Subjective Units of Distress Scale (a visual analog scale scored from 0 to 100 for the assessment of current subjective level of distress) were performed every 5 minutes until three successive ratings were unchanged, indicating that the subject had adapted to the study setting. Baseline subjective ratings were then collected, including a 17-item PTSD symptom scale
(35), Clinician Administered Dissociative States Scale, a reliable and valid 27-item scale for the measurement of current dissociative states
(36), another Subjective Units of Distress Scale, and a visual analog scale (scored from 0 to 100) for the assessment of fear
(35).
Subjects then underwent scanning during readings of neutral and traumatic (related to personalized childhood sexual abuse events) scripts. All scripts were 1 minute in length and were read aloud in a normal tone of voice by the research associate who had helped the subjects prepare the traumatic scripts. First, subjects underwent two scans while listening to two different neutral narratives, during which they were instructed to count the number of times they heard the letter d (data not presented here). Then subjects underwent two scans, during which they listened to two different neutral narratives preceded by instructions to form an image in their mind of the narrative being read and to try to remember as much of the scene as they could. Subjects then underwent two scans, during which they listened to two personalized scripts of their own childhood sexual abuse event; the scripts were again preceded by instructions to form an image in their mind of the narrative being read and to try to remember as much of the scene as they could. A fixed order (neutral scripts followed by childhood abuse scripts) was used for all subjects in order to prevent anxiety elicited by the traumatic scripts from persisting into the neutral scripts.
According to the logic of the study design, differences in brain blood flow between the childhood sexual abuse scripts and the neutral scripts would be secondary to the specific effects of memories of childhood abuse, controlling for other factors, including attention, auditory perception, and comprehension of a coherent verbal narrative.
Ten seconds before administration of [15O]H2O, subjects received instructions to form an image in their mind of the narrative being read and to try to remember as much of the scene as they could. This was followed by the beginning of the reading of the script, which was 60 seconds in duration. At the same time of the beginning of the reading of the script, subjects received a bolus injection of 30 mCi of [15O]H2O, followed 10 seconds later by a PET scan acquisition that was 80 seconds in length. The onset of the PET scan acquisition was timed to correspond to the point of maximum rate of increase in uptake of tracer into the brain. The script was timed to effect maximum levels of PTSD symptoms at the time of maximal uptake of tracer in the brain. With the bolus injection method of [15O]H2O (which has a half-life of 110 seconds), tracer peaks at 10 seconds, with 90% of counts obtained in the first 60 seconds after peak, which is the time during which the scripts were read. In addition, a state of increased PTSD symptoms is expected in the 20 seconds after the end of the script reading. PET imaging was performed with a Posicam PET camera (Positron Corp.) (in plane resolution after filtering, 6-mm full width at half maximum). Subjects then underwent behavioral ratings for the time of the presentation; measures included the Panic Attack Symptom Scale, Clinician Administered Dissociative States Scale, Subjective Units of Distress Scale, fear analog, and PTSD Symptom Scale.
Images were reconstructed and analyzed on a SunSparc workstation through use of statistical parametric mapping (spm96). Images for each patient set were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration of each slice and was adjusted to a nominal value of 50 ml/minute per 100 g. The data underwent transformation into a common anatomical space and were smoothed with a three-dimensional Gaussian filter to 16-mm full width at half maximum. Regional blood flow, with global blood flow as a covariate, was compared between childhood abuse and neutral script conditions for the women with and without PTSD. The interaction between group (PTSD versus non-PTSD) and condition (childhood abuse versus neutral scripts) was also examined. Statistical analyses yielded image data sets in which the values assigned to individual voxels correspond to the t statistic
(37). Statistical images were displayed with values of z score units. A threshold z score of 3.09 (p<0.001) was used to examine areas of activation within hypothesized areas. The threshold of z score corresponds to a p value of <0.001 for a one-tailed t test. Since the current study was a replication of a prior study of combat veterans with PTSD, the direction of change in blood flow was hypothesized a priori, which justifies the use of a one-tailed test. Spm96 employs a fixed-effects analysis that may limit the ability to generalize from the study population; the methodology for mixed random effects has not been published in the literature. Location of areas of activation was identified as the distance from the anterior commissure in millimeters, with x, y, and z coordinates; a standard stereotaxic atlas was used
(38).
Behavioral measures (PTSD, Clinician Administered Dissociative States Scale, Subjective Units of Distress Scale, and analog rating scores) were compared for PTSD and comparison subject groups through use of repeated measures analysis of variance, with behavioral state over time (baseline and neutral and traumatic script periods) as the repeated measure.
RESULTS
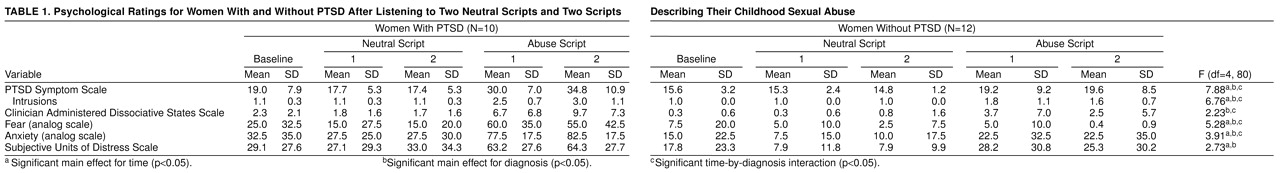
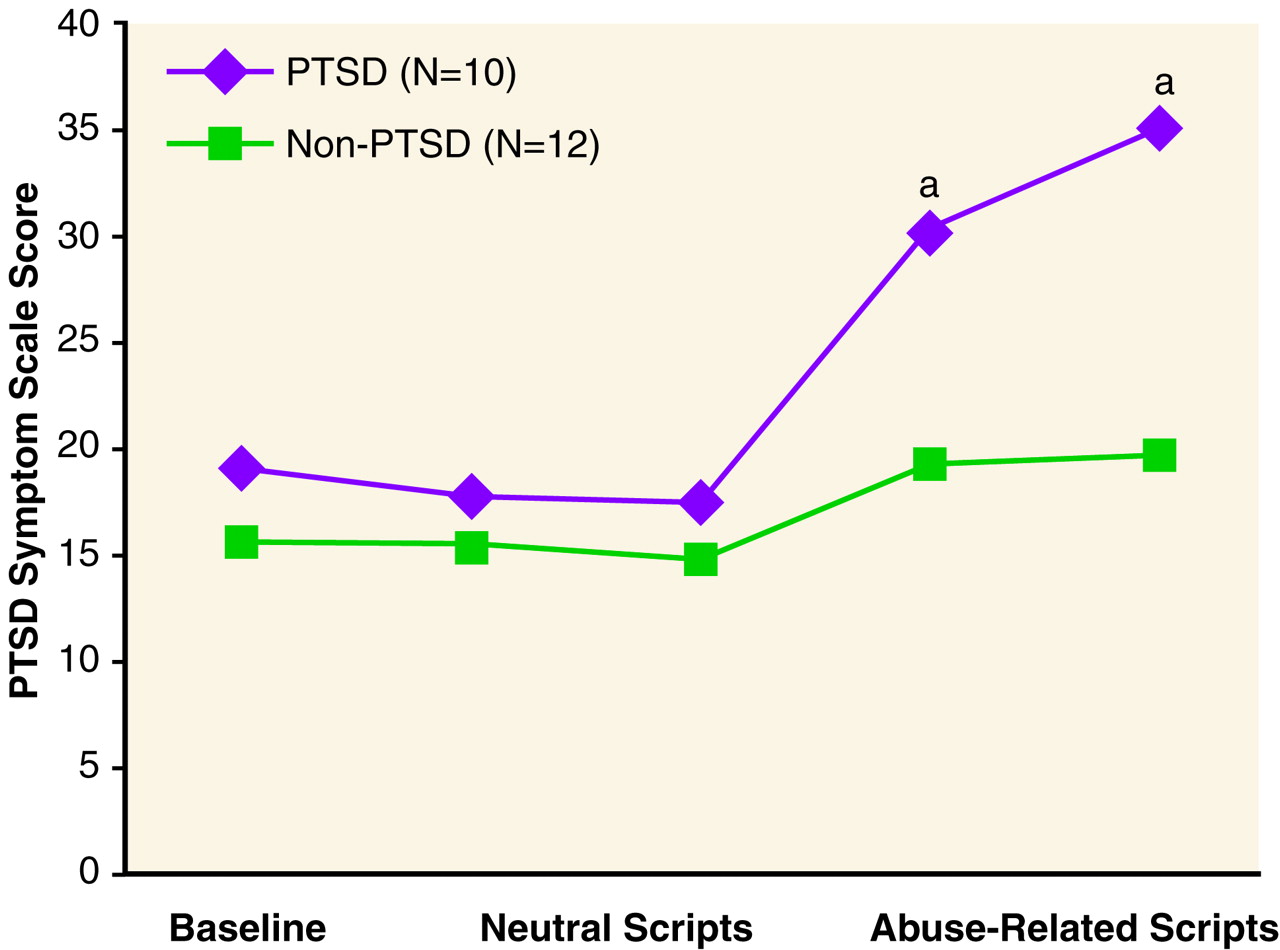
Sexually abused women with PTSD had greater responses to scripts than sexually abused women without PTSD for PTSD symptoms (
Figure 1), as well as for symptoms of anxiety and fear (
table 1).
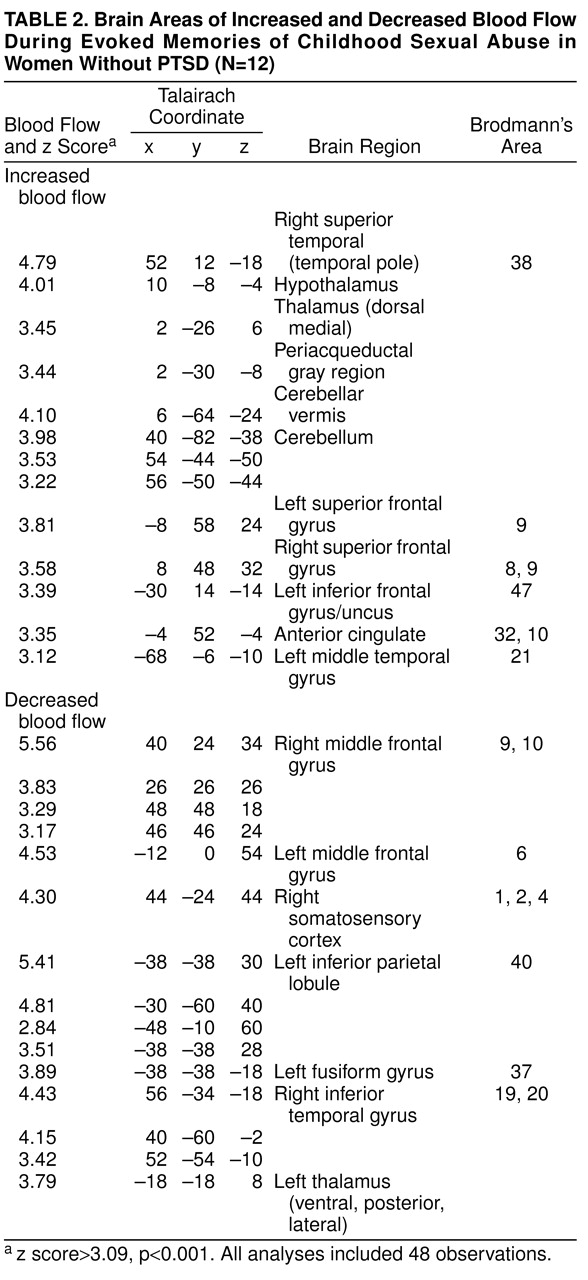
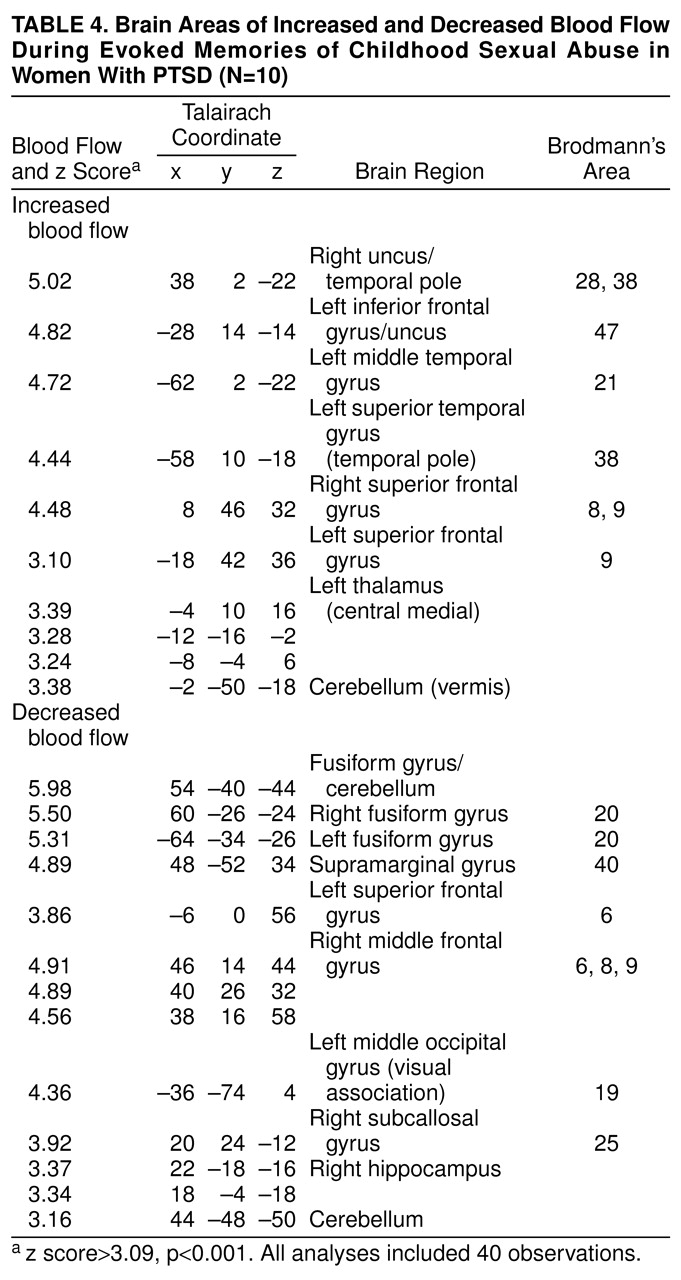
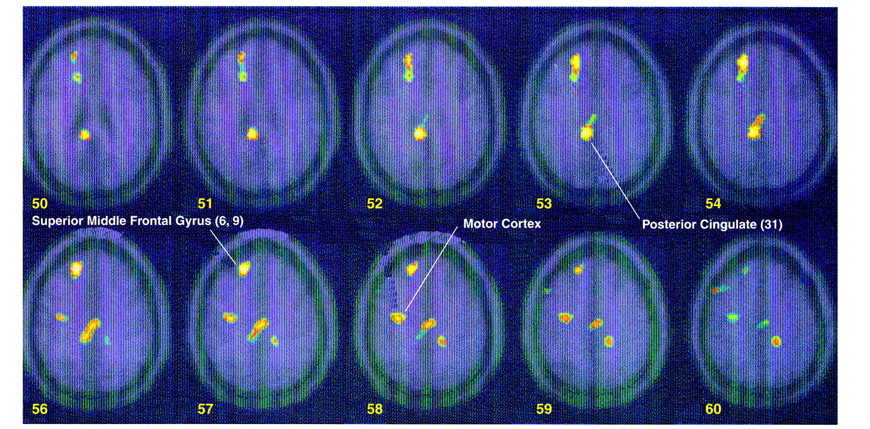
Exposure to scripts of childhood sexual abuse resulted in increased blood flow in posterior cingulate (area 31) and anterolateral prefrontal cortex (superior and middle frontal gyri bilaterally, areas 9 and 10) in women with PTSD (z score>3.00, N=40, p<0.001) (
table 2) but not in women without PTSD (
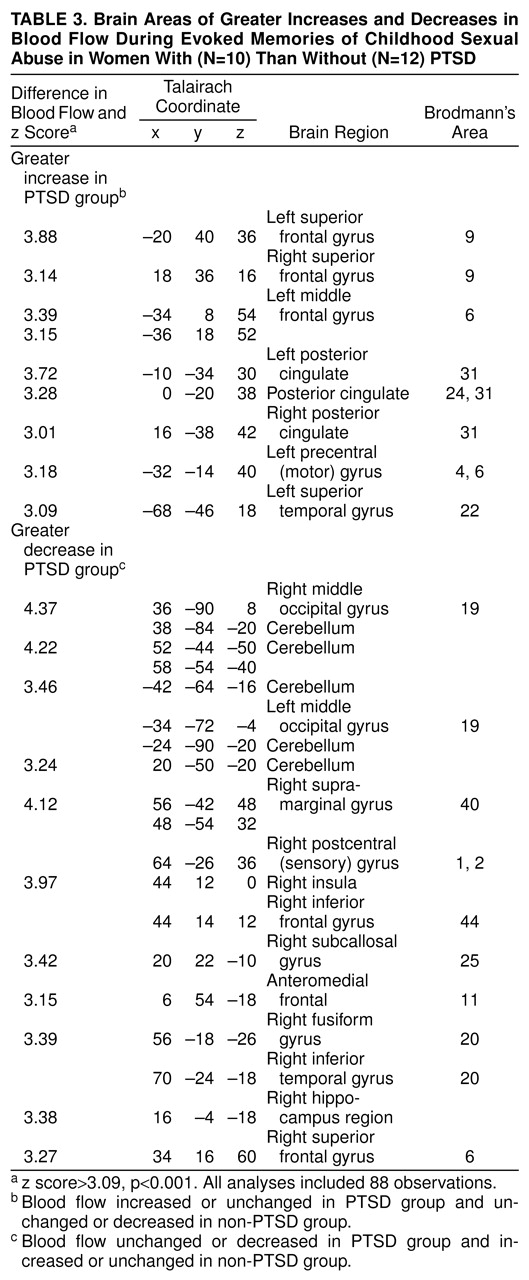
table 3). Direct comparison of activation between women with and without PTSD showed that these patterns of activation were greater in the PTSD group (i.e., significant group-by-task interaction) (
table 4,
Figure 2). There were also significant differences between the groups in left motor cortex (Brodmann’s areas 4 and 6) (z score>3.00, N=88, p<0.001) (
table 4,
Figure 2) (Brodmann’s areas here and throughout are approximate). Women with PTSD had decreased blood flow in medial prefrontal cortex (subcallosal gyrus [area 25], which is a component of the inferior portion of anterior cingulate), with a failure of activation in an adjacent portion of anterior cingulate, area 32 (
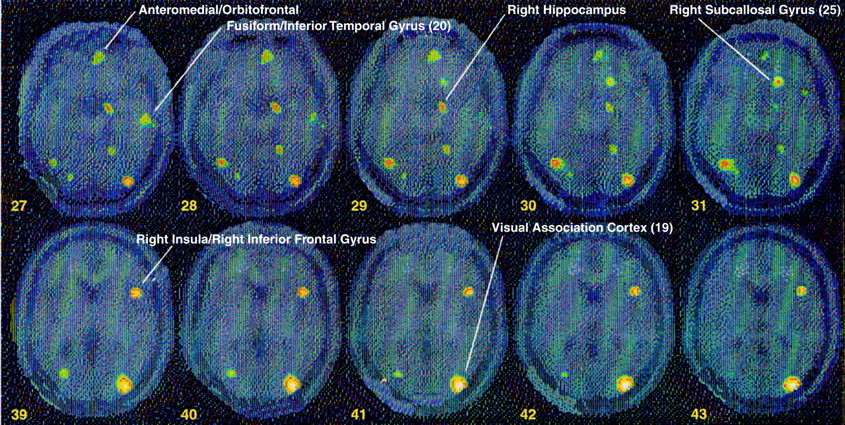
table 2 and
table 4). There was also decreased blood flow in a portion of parietal cortex (supramarginal gyrus [area 40]), right hippocampus, visual association cortex (area 19), fusiform gyrus/inferior temporal gyrus (area 20), and dorsolateral prefrontal cortex (areas 6 and 8) (z score>3.00, N=40, p<0.001) (
table 3). Again, contrasts between the non-PTSD and PTSD groups showed significant differences between the groups in these areas (
table 4,
Figure 3).
Activation in cerebellum, thalamus, uncus, left inferior frontal gyrus, and temporal pole with memories of childhood sexual abuse was nonspecifically seen in both women with and without PTSD (
table 2 and
table 3).
DISCUSSION
While listening to scripts of childhood sexual abuse events, women with PTSD related to childhood sexual abuse demonstrated increased activation in posterior cingulate, anterolateral prefrontal cortex (Brodmann’s areas 6 and 9), and motor cortex. Women with PTSD showed a deactivation in subcallosal gyrus region of anterior cingulate (area 25), with a failure in activation in adjacent anterior cingulate (area 32), as well as deactivation in right hippocampus, fusiform/inferior temporal gyrus, supramarginal gyrus, and visual association cortex. These changes were greater than those seen in sexually abused women without PTSD. Both sexually abused women with and without PTSD showed activation in cerebellum, temporal pole, left inferior frontal gyrus, and thalamus, suggesting that this is a generalized neural response to memories of upsetting childhood sexual abuse experiences that is not specific to the pathological state of PTSD.
These findings are consistent with our previous study of combat veterans with PTSD who were exposed to combat-related slides and sounds
(25), as well as with findings from other PET studies. In both studies we found increased activation in posterior cingulate and motor cortex, failure of activation in anterior cingulate, and decreased blood flow in subcallosal gyrus and visual association cortex with traumatic stimuli in subjects with PTSD relative to subjects without PTSD. Posterior cingulate has been found to play a role in emotional processing in normal subjects, as measured by response to watching a film of a bank robbery
(39) or listening to threat-related words
(40). In addition, increased blood flow in right posterior cingulate was found in normal subjects listening to highly personal autobiographical events
(41), which is similar to listening to autobiographical traumatic events in the current study. Posterior cingulate also plays an important role in visuospatial processing
(6,
8) and is therefore an important component of preparation for coping with a physical threat, a response that is heightened in PTSD
(42). Motor cortex activation may represent the neural correlate of preparation for action. Parietal cortex is involved in spatial memory and visuospatial processing
(43). In our prior study
(25) we found greater activation in left inferior parietal lobule in subjects with PTSD, whereas the current study showed decreased blood flow in an adjacent parietal area, right supramarginal gyrus. Parietal lobe is involved in modulation of arousal
(44), and lesions in the right inferior parietal lobule were correlated with impairments in identification of negative emotion
(45). Increased blood flow was also found in prefrontal cortex in the current study. Middle/inferior frontal gyrus has been implicated in encoding and retrieval of verbal memories, with several studies showing a lateralization for encoding on the left and retrieval on the right
(46). We found increased function in this area in PTSD, which may represent a neural correlate of the strength of remembrance in the PTSD group. The areas reviewed previously (motor, parietal, prefrontal, and visual association cortex, and posterior cingulate) are functionally linked and may operate together in the mediation of traumatic remembrance in PTSD patients
(4,
16).
A number of PET studies have implicated medial prefrontal cortex in stress and emotion
(47–
51). We found dysfunction in this area in two studies of PTSD patients, whereas failure of activation or decreased baseline function was found in a meta-analysis of patients with anxiety disorders
(23) and in patients with depression
(52,
53). This area has been implicated in emotion and social behavior
(10) as well as regulation of the peripheral glucocorticoid and sympathetic response to stress
(6,
8,
54). Medial prefrontal cortex also has inhibitory connections to the amygdala
(6,
8,
54–
56) that play a role in extinction of fear responding
(9,
57). Dysfunction of medial prefrontal cortex may represent a neural correlate of the failure of extinction to fear responding, as well as other pathological emotions in PTSD.
Decreased blood flow was seen in right hippocampus in women with PTSD during exposure to scripts of childhood sexual abuse. Multiple studies now have shown smaller hippocampal volume and deficits in declarative memory function consistent with impaired hippocampal function in PTSD
(18,
19,
26,
27), whereas animal studies have implicated the hippocampus in emotional responses to the context of a stressful environment
(12,
13). In a previous PET study we found decreased metabolism in hippocampus with yohimbine (which stimulates brain norepinephrine release) in PTSD
(20). We also found decreased blood flow in visual association cortex (middle occipital gyrus, area 19) in women with PTSD, whereas women without PTSD had an increase in blood flow in this area, during script-induced remembrance of childhood sexual abuse. Women with PTSD may attempt to dispel visual images of upsetting events to a greater extent than sexually abused women without PTSD. We also found deactivation of another brain area involved in complex visual processing, fusiform gyrus/inferior temporal gyrus, that is specifically involved in memory of faces. Decreased blood flow in this area may have occurred in PTSD for reasons that are similar to those for visual association cortex.
As previously shown in a variety of anxiety-inducing techniques, exposure to traumatic scripts resulted in a nonspecific activation in cerebellum, thalamus, and temporal pole that was seen in both PTSD and non-PTSD subjects
(23,
25,
51,
58). Cerebellum has important connections to anterolateral prefrontal cortex via thalamus that mediate a role in cognition that has been underappreciated
(59,
60). Temporal pole (Brodmann’s area 38) has been implicated in autobiographical memory in normal subjects
(41).
The current study did not demonstrate activation of the amygdala, a brain area that has been implicated in conditioned fear responses in animals. One prior PET study showed activation of an area involving right amygdala and insula in PTSD patients exposed to traumatic scripts
(22). Because of the absence of a control group in that study, it is not possible to say that increased amygdala activation is specific to PTSD. The authors also pointed out that because of limitations of PET resolution and the small size of the amygdala, the activation could be related primarily to insular activity, and they suggested that the finding should be replicated. Reiman et al.
(50) argued that in healthy subjects, amygdala is activated by exteroceptive emotional cues rather than recall, consistent with present findings. PET studies in normal subjects have shown that amygdala activation during encoding of emotional films was correlated with recall of encoded material. The authors argued that on the basis of their data, the amygdala was involved in encoding of the emotional significance of events but not in recall per se
(61). This would be consistent with the current study and our prior report in which recall of traumatic events was not associated with increased amygdala activation. Inconsistent findings have also been found related to fear-conditioned startle in PTSD, a physiological response that is known in animals to be mediated by the central nucleus of the amygdala. Although fear conditioning is a compelling model for the anxiety disorders, it may be an oversimplistic model for the complexity of pathological emotions in PTSD.
One important question in the PTSD field is why different people exposed to the same level of stress do not all develop PTSD. One factor that has been proposed to explain this fact is that different levels of vulnerability explain susceptibility to the development of PTSD. The current study does not answer the question of whether differences in level of vulnerability to PTSD underlie differences in our PTSD and non-PTSD groups or differences in the disorder per se. In addition, the PTSD group had higher sexual abuse severity scores on the Early Trauma Inventory, even though both groups were exposed to arguably severe trauma (rape or molestation before age 18), so the differences between the groups may be attributable to differences in severity of exposure to trauma.