Dysfunction or dysregulation of neurotransmission mediated by the
N-methyl-
d-aspartate (NMDA) receptor may contribute to the pathophysiology of schizophrenia
(1). To test this hypothesis, potentiation of NMDA receptor-mediated neurotransmission has been examined as a treatment for schizophrenia. Three agonists at the glycine site on the NMDA receptor have been examined thus far:
d-serine
(2),
d-cycloserine
(3), and glycine
(4), which have all been shown to reduce negative symptoms and variably improve cognitive impairment in antipsychotic-treated schizophrenic patients.
d-Serine also improved positive and cognitive symptoms
(2).
Notably, when
d-cycloserine was added to clozapine, it actually caused a deterioration in negative symptoms
(5). The apparent antagonistic effects of the partial agonist under these conditions suggest that clozapine reduces negative symptoms by augmenting NMDA receptor function. To test this hypothesis, we have examined the effects of
d-serine, a full agonist at the glycine site on the NMDA receptor, in patients stabilized on regimens of clozapine. We hypothesized that the full agonist
d-serine would not exacerbate negative symptoms, unlike the partial agonist
d-cycloserine, and that if clozapine has fully activated the NMDA receptor glycine site,
d-serine should not have additional therapeutic effects.
METHOD
Patients were recruited from the inpatient unit of Tsyr-Huey Mental Hospital, which is affiliated with Kaohsiung Medical School, Kaohsiung, Taiwan. After a description of the study to the patients, written informed consent was obtained.
Interviews with the Structured Clinical Interview for DSM-IV were conducted to determine diagnoses, and all enrolled patients fulfilled the DSM-IV criteria for a diagnosis of schizophrenia. Patients with axis I diagnoses other than schizophrenia, significant depressive symptoms, or serious medical or neurological illness were excluded. The patients had not responded to conventional antipsychotics and were classified as treatment resistant according to the definition of Kane et al.
(6). They also all fulfilled the criteria for primary deficit syndrome
(7) and had a score of 45 or higher on the Scale for the Assessment of Negative Symptoms (SANS).
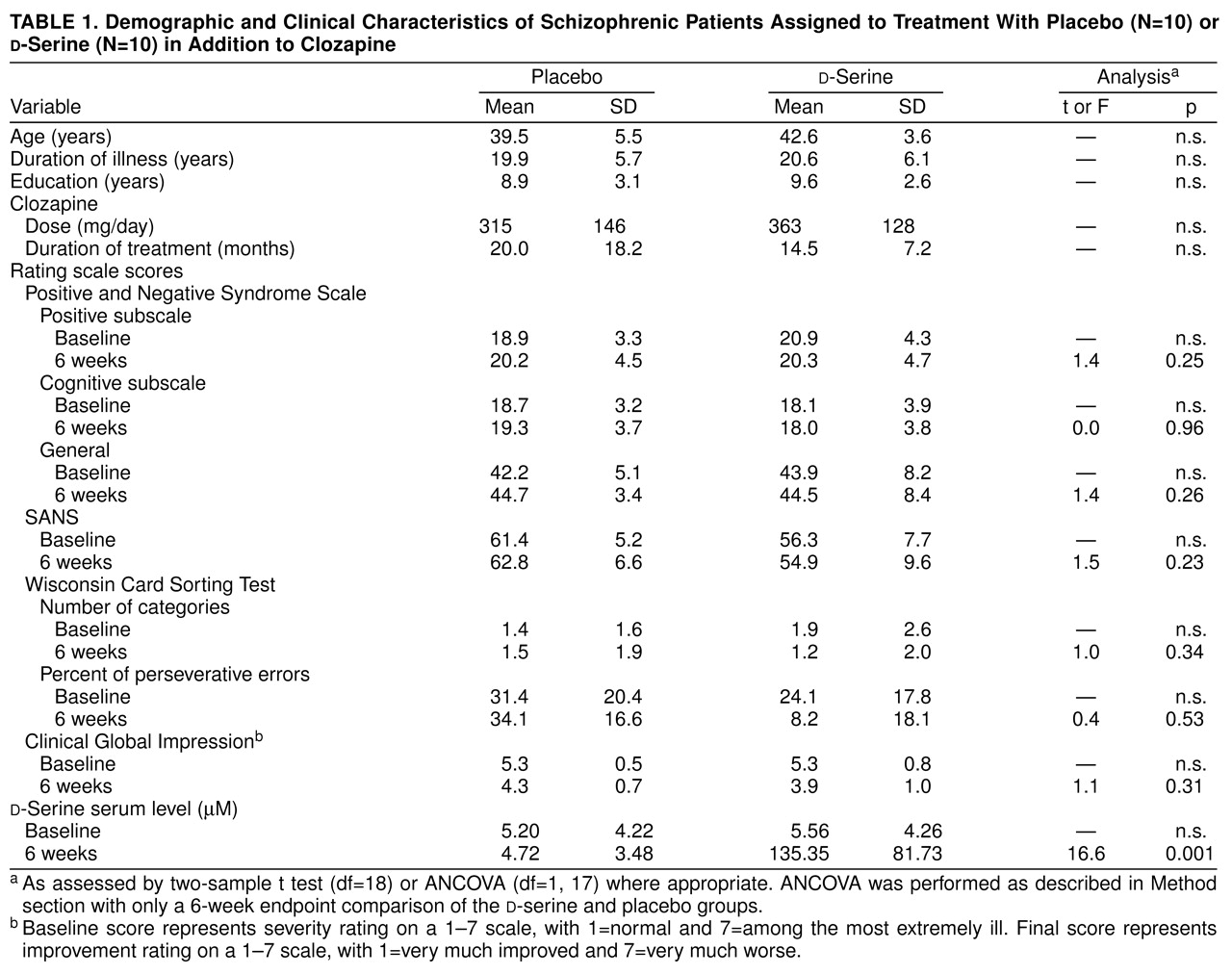
Their clozapine doses were stable for at least 3 months before enrollment in the study and remained the same during the
d-serine trial (
table 1). The clinical effect of clozapine was stable, as indicated by no change in score on the Clinical Global Impression (CGI) or less than 5% change on the Brief Psychiatric Rating Scale for at least 6 weeks. Twenty schizophrenic patients enrolled in and completed the 6-week random-assignment, double-blind, placebo-controlled study of
d-serine at a dose of 30 mg/kg per day (
table 1).
The assessment tools were the same as in the previous study of
d-serine for nonclozapine patients
(2). The following ratings were performed at baseline and biweekly thereafter: CGI, Positive and Negative Syndrome Scale positive and cognitive subscales, SANS, and Hamilton Depression Rating Scale. To minimize learning effects, the Wisconsin Card Sorting Test was administered only at baseline and at the end of 6 weeks of treatment.
Biweekly side effect assessments included the Simpson-Angus Rating Scale, Abnormal Involuntary Movement Scale (AIMS), and Barnes’s Rating Scale for Drug-Induced Akathisia. Systemic side effects were reviewed by applying the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale. As in the previous study
(2), we also obtained fasting-state blood samples to determine the trough serum level of amino acids and
d-serine by high-performance liquid chromatography-fluorescent detection.
Demographic characteristics of the d-serine and placebo groups were compared by means of Student’s two-sample t test for continuous variables and chi-square test for categorical variables. For assessment of efficacy, serum levels, and side effects, we performed analyses of covariance (ANCOVAs) with baseline (week 0) values as covariates to determine the significance of observed treatment effects. An alpha level of 0.05 and two-sided statistical tests were used throughout the analysis.
RESULTS
The sex ratios (female:male) of the two groups were similar: 5:5 for placebo and 4:6 for
d-serine (χ
2=0.20, df=1, n.s.). Other demographic and illness characteristics were also similar (
table 1). No clinical measures showed significant improvement at week 2, 4, or 6 with
d-serine treatment (CGI: F=1.43, df=3, 15, p=0.27; Positive and Negative Syndrome Scale positive subscale: F=1.10, p=0.38; SANS: F=0.45, p=0.72; Positive and Negative Syndrome Scale cognitive subscale: F=1.02, p=0.41). The number of categories finished in the Wisconsin Card Sorting Test (F=0.91, df=1, 17, p=0.36) or the percentage of perseverative errors (F=0.92, p=0.35) did not indicate a significant improvement in the
d-serine group.
No significant reduction in symptoms of any type was present in the placebo group (
table 1). The scores on the Positive and Negative Syndrome Scale general subscale (F=0.57, df=3, 15, p=0.64) and Hamilton depression scale (F=0.93, p=0.45) were not significantly different after 6 weeks of
d-serine or placebo treatment (
table 1).
Serum levels of amino acids and
d-serine were similar at baseline in the two groups (data not shown). ANCOVA indicated that the
d-serine and placebo groups did not differ at week 2, 4, or 6 in serum level of aspartate, glutamate,
L-serine, or glycine (data not shown). In the
d-serine group,
d-serine levels were elevated from baseline at week 2, and the elevation persisted into weeks 4 and 6 (
table 1).
The scores on the Simpson-Angus Rating Scale, AIMS, and Barnes’s Rating Scale for Drug-Induced Akathisia were similar in the two groups (data not shown). These side effect measures did not change in either the placebo or d-serine treatment group. Also, the side effect measures did not differ between groups throughout the 6 weeks (Simpson-Angus Rating Scale: F=0.0, df=3, 15, p=1.00; AIMS: F=0.0, p=1.00; Barnes’s Rating Scale for Drug-Induced Akathisia: F=0.0, p=1.00). There were no significant treatment-emergent adverse events in either the placebo or d-serine group. The results of routine blood cell counts and blood chemistry analyses after 6 weeks of d-serine or placebo treatment remained unchanged and were all within the normal ranges (data not shown).
DISCUSSION
Our findings indicate that, in contrast to patients treated with other antipsychotics
(2), patients treated with clozapine do not experience improvement in schizophrenic symptoms when also given
d-serine. The NMDA glycine site agents
d-serine, glycine, and
d-cycloserine all improved negative symptoms in patients treated with nonclozapine antipsychotics, whereas no agent has yet demonstrated any therapeutic effect for clozapine-treated patients
(2–
5,
8).
As a partial agonist,
d-cycloserine may antagonize NMDA receptor neurotransmission when glycine or other agonist(s) saturates the NMDA receptor glycine site. Consistent with this, the studies of
d-cycloserine in schizophrenic patients revealed a U-shaped dose-response curve, and doses of 250 mg/day or greater worsened both positive and negative symptoms
(9–
11). This mechanism had been proposed to account for the exacerbation of symptoms caused by
d-cycloserine in schizophrenic patients treated with clozapine
(5). Some nonclozapine antipsychotics may also have partial agonist activity at the NMDA receptor glycine site
(12), which can facilitate the antagonistic effect of
d-cycloserine when it is administered with nonclozapine antipsychotics.
Since
d-serine is a full agonist for the NMDA receptor glycine site, maximal activation of this site can be achieved without the concern of antagonism that a partial agonist can induce. Nevertheless, in patients receiving clozapine, no therapeutic effect of
d-serine was observed. This lack of efficacy is unlikely to be due to the pharmacokinetic difference in the clozapine-treated patients; the levels of
d-serine in the clozapine-treated patients (
table 1) were comparable to those found in non-clozapine-treated patients
(2).
There are several possibilities as to why
d-serine, and glycine, do not improve symptoms in clozapine-treated patients. First, the lack of effects of
d-serine might be due to the antipsychotic status. Clozapine may have agonist, or partial agonist, activity at the NMDA receptor, contributing to its unique clinical efficacy. Hence, the addition of
d-serine could not further enhance the NMDA neurotransmission already influenced by clozapine. Heresco-Levy et al.
(4) reported that high-dose glycine improved the negative symptoms of rigorously defined treatment-resistant patients, suggesting that treatment-resistant patients do respond to an NMDA receptor glycine site agent. Potkin et al.
(8) reported that glycine supplementation did not improve the symptoms of clozapine-treated patients; in addition, they suggested that glycine interfered with the antipsychotic efficacy of clozapine. This finding is consistent with a partial agonist activity of clozapine and explains why
d-serine cannot further improve the symptoms of clozapine-treated patients.
Second, in current clinical practice patients treated with clozapine often are older, have longer durations of illness, have had several prior antipsychotic exposures, and represent a subpopulation of severe pathology. Rigorously defined treatment resistance in the patients in the present study indicates that they had more severe pathology (as compared to the patients in the previous study of
d-serine, who were receiving nonclozapine antipsychotics
[2]) and might therefore be resistant to other treatment, including
d-serine and glycine. Nevertheless, the differential efficacy of
d-serine, glycine, and
d-cycloserine for non-clozapine-treated versus clozapine-treated patients does not support this hypothesis, since all three NMDA glycine site agents can improve the negative symptoms of deficit or treatment-resistant patients treated with nonclozapine antipsychotics
(2–
4).
Power analysis indicated that we would likely have observed an improvement if the effect size in this study were similar to that in the previous study (for SANS: d=1.4, alpha=0.05, N=10, power=0.84
[2]). However, a larger study group might reveal a small effect of
d-serine in clozapine-treated patients. Also, doses different from what we used might mitigate the partial agonist effect of clozapine and produce symptom improvement exceeding the clinical benefits accomplished by clozapine.