Tardive dyskinesia, by definition, occurs late in the course of neuroleptic treatment. The minimum duration of neuroleptic use necessary to produce tardive dyskinesia is usually thought to be 3 months
(1), although DSM-IV criteria specify that this may be 1 month in individuals 60 years of age or older. Two studies
(2,
3) have reported a cumulative annual incidence of tardive dyskinesia of greater than 25% among older patients; however, we found no published studies of the incidence of tardive dyskinesia among neuroleptic-naive patients after 1 and 3 months of treatment and no studies comparing the 12-month cumulative incidence of tardive dyskinesia in patients with a history of receiving neuroleptics for 30 days or less compared with those receiving neuroleptics for more than 30 days. Therefore, we undertook the present study of the incidence of tardive dyskinesia among middle-aged and elderly outpatients.
METHOD
The subjects were 307 psychiatric outpatients; all provided informed consent. None of the subjects had tardive dyskinesia at baseline, and as a group the duration of their total lifetime use of neuroleptic medication was 5 years or less. All of the patients were treated with conventional (but not atypical) antipsychotics during at least a portion of the study. A previous report
(3) provided data on 189 of these 307 patients. The patients were recruited from a variety of sources, but most were from the San Diego Veterans Affairs Medical Center or the University of California, San Diego, Medical Center. The majority of the patients were men (N=248, 80.8%) and Caucasian (N=252, 82.1%). Their mean age was 66.2 years (SD=12.2).
The patients’ DSM-III-R-based psychiatric diagnoses were dementia (N=98, 31.9%), schizophrenia (N=51, 16.6%), schizoaffective disorder (N=10, 3.3%), delusional disorder (N=5, 1.6%), “organic” psychoses (N=34, 11.1%), psychotic disorder not otherwise specified (N=13, 4.2%), mood disorders (N=48, 15.6%), and other (N=48, 15.6%). All patients were prescribed conventional neuroleptics for psychotic or other severe behavioral disturbances. The two most commonly used neuroleptics were haloperidol and thioridazine: 135 (44.0%) of the patients were treated with haloperidol, and 52 (16.9%) were treated with thioridazine. We used the following formulae for dose equivalence: 2.4 mg of haloperidol or 111.1 mg of thioridazine were considered equivalent to 100 mg of chlorpromazine
(4). The median neuroleptic dose prescribed at baseline was 68.4 mg/day of chlorpromazine equivalent
(4). Forty-five (14.7%) of the patients received anticholinergics at baseline, usually at low doses.
The Abnormal Involuntary Movement Scale (AIMS)
(5) was used to assess dyskinesia at baseline and 1, 3, 6, 9, and 12 months later. Tardive dyskinesia was diagnosed by using Schooler and Kane’s criteria
(1) except for an absence of the required minimum duration of neuroleptic treatment. Additional assessments included measures of global neurocognitive status (Mini-Mental State
[6]), depressive symptoms (Hamilton Depression Rating Scale
[7]), and extrapyramidal symptoms (modified Simpson-Angus Rating Scale
[8]).
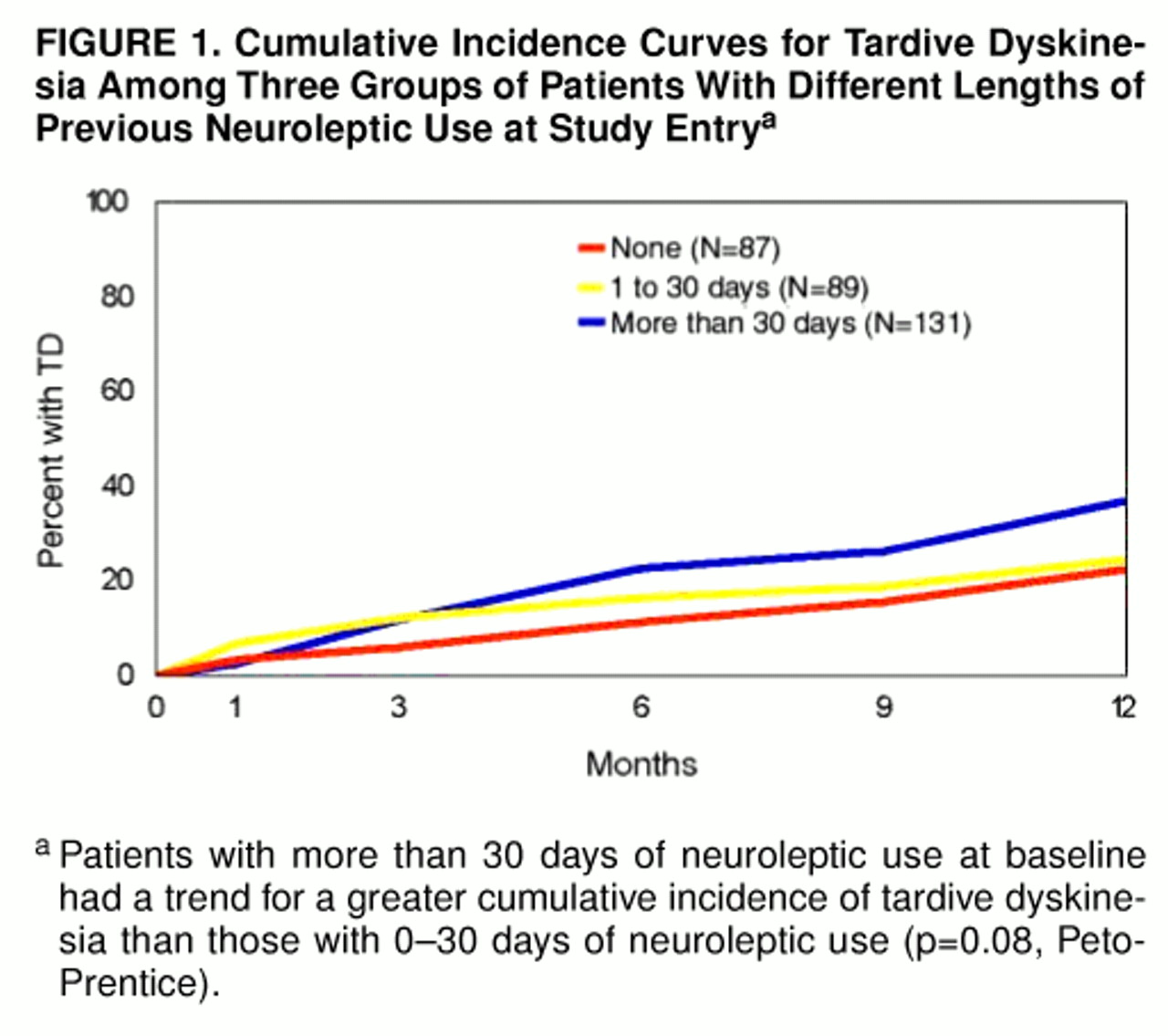
For purposes of analysis, subjects were classified into three groups on the basis of the duration of their previous use of neuroleptics: 1) neuroleptic-naive (N=87), 2) 1–30 days of previous use (N=89; median=10.0 days) and 3) more than 30 days of previous use (N=131; median=115.0 days).
The cumulative incidence of tardive dyskinesia was calculated by using life-table survival analysis
(9), and specific risk factors were determined by using Cox regression analysis
(10).
RESULTS
For the neuroleptic-naive group, the mean cumulative incidence of tardive dyskinesia was 3.4% at 1 month (95% confidence interval=0.0% to 7.3%) and 5.9% at 3 months (95% confidence interval=0.9% to 10.9%). There was no significant difference in the 12-month cumulative incidence of tardive dyskinesia between patients who were neuroleptic-naive (22.3%) and those with 1–30 days of total lifetime neuroleptic use at baseline (24.6%) (p=0.36, Peto-Prentice). Therefore, we combined these two groups for subsequent analyses. The patients with more than 30 days of previous neuroleptic tended to have a greater 12-month cumulative incidence of tardive dyskinesia (36.9%) than those with 0–30 days of neuroleptic use (p=0.08, Peto-Prentice) (figure 1).
The groups with 0–30 days versus more than 30 days of previous neuroleptic use were similar in sex, ethnicity, presence of diabetes, history of alcohol abuse or dependence, and Hamilton depression scale and modified Simpson-Angus scale scores at baseline. The groups differed, however, in other respects. Patients with 0–30 days of neuroleptic use were older, were more likely to have a diagnosis of dementia or organic mental syndrome, had lower Mini-Mental State and global AIMS scores at baseline, received lower daily neuroleptic doses, and were less likely to receive anticholinergics. To determine if these group differences contributed to the differential risk of tardive dyskinesia, the following variables were used in a Cox regression: age, diagnostic type (“organic” versus primary psychiatric disorder), Mini-Mental State score, global AIMS score, duration of previous neuroleptic use (0–30 days versus more than 30 days), daily neuroleptic dose, and use of anticholinergics. The only significant predictor of tardive dyskinesia risk in this model was duration of previous neuroleptic use at study entry.
We compared the 12-month cumulative incidence rate of the previously reported 189 patients
(3) with that of the 118 new patients. There was no significant difference in tardive dyskinesia incidence (p=0.78, Peto-Prentice). Twelve-month mean cumulative incidence rates for tardive dyskinesia in the total study group (N=307) were 34.1% in patients younger than 60 years of age, and 27.1% in those 60 years old or older. Among patients who were 60 years old or older, the 12-month mean cumulative incidence rates for tardive dyskinesia for patients with versus those without dementia were 24.9% and 30.2%, respectively. These differences were not significant. The nonsignificantly lower rates in patients who were 60 years old or older and in patients with dementia seemed to be related to lower neuroleptic doses and duration of previous use in those groups. In terms of severity of tardive dyskinesia, the breakdown of the 62 tardive dyskinesia patients by total AIMS score at the time of initial diagnosis of tardive dyskinesia was as follows: 30 (48.4%) had total scores of 5 or less; 27 (43.5%) had scores of 6 to 8; and five (8.1%) had scores of 9 or higher.
DISCUSSION
We studied outpatients being treated with relatively low doses of typical neuroleptics. Our findings suggest that a substantial proportion of middle-aged and elderly patients develop tardive dyskinesia relatively early in the course of neuroleptic treatment. The cumulative incidence of tardive dyskinesia after only 3 months of treatment was 5.9%, whereas younger adults need at least 1 year of neuroleptic use for a 4% to 5% cumulative incidence
(11). Therefore, one would be able to determine the relative risk of tardive dyskinesia associated with typical versus atypical antipsychotics among older patients by using relatively short-term longitudinal studies.
One limitation of our study was the absence of a control group of older patients with similar diagnoses who did not receive neuroleptics. Given the elevated risk of spontaneous dyskinesia in older patients
(12), it is conceivable that some of the subjects in our study might have developed dyskinesia even in the absence of neuroleptic use. It is impractical, however, to have a true control group because it would be unethical to deny the use of neuroleptics to patients who need them for controlling their psychotic or severe behavioral disturbances. Nevertheless, it is highly unlikely that a large proportion of patients would develop dyskinesia spontaneously over a relatively short period of time.
Given that some of our subjects developed tardive dyskinesia after only 1 month of neuroleptic use, the DSM-IV criterion of 1 month, instead of the usual 3 months, of minimum neuroleptic use for a diagnosis of tardive dyskinesia in elderly patients should be broadened to include middle-aged patients (45–60 years).