The study of risk factors for tardive dyskinesia has assumed greater importance in recent years with the increasing proportion of elderly patients, a group particularly vulnerable to abnormal movements. Age-related medical illnesses may play a role in the higher rate of tardive dyskinesia among the elderly. For example, there is evidence that neuroleptic-treated patients with diabetes mellitus may have a higher rate of tardive dyskinesia than those without diabetes
(1) and that fasting blood sugar levels are higher among patients with tardive dyskinesia
(2). Particularly fascinating is a report by Mukherjee and Mahadik
(3) describing a greater incidence of diabetes in family members of patients with tardive dyskinesia, suggesting that abnormalities in dopaminergic mechanisms and glucose control may be fundamentally related at a genetic level. In contrast, other large studies have not found an association between tardive dyskinesia and diabetes, even in samples of older subjects
(4). Therefore, the relationship between glucose tolerance and abnormal movements remains controversial.
In the study reported here, we hypothesized that among chronically treated patients with schizophrenia who were taking neuroleptic medications, patients with impaired glucose tolerance would be more likely to have abnormal movements than those with normal glucose tolerance. We also expected that a measure of insulin resistance (i.e., insulin level relative to concomitant glucose level) would correlate with the severity of abnormal movements.
METHOD
Twenty-one patients were recruited from consecutive admissions to the Mental Health Clinical Research Center at the University of Iowa. All subjects signed written informed consent after the protocol and procedures had been fully explained. All subjects had DSM-IV-diagnosed schizophrenia and been chronically ill for a minimum of 20 years. Patients with dementia, head trauma, or severe substance abuse were excluded. Patients were stable on their usual neuroleptic medication regimens at the time of assessment.
Patients were given an oral glucose tolerance test after an overnight fast. Fasting blood glucose and insulin levels were obtained at the start of the testing period. The patients then were given a 75-gm glucose load of commercial carbohydrate. Blood samples were collected hourly for 2 hours. Serum glucose measures (mg/dl) were determined by using mass spectrophotometry, and insulin concentrations (µU/ml) were determined by using radioimmunoassay
(5). Abnormal movements were rated by using the Abnormal Involuntary Movement Rating Scale (AIMS)
(6). The movement ratings were obtained by a trained research nurse in the morning before laboratory testing on two separate occasions, allowing a mean score to be generated for each patient.
The presence of impaired glucose tolerance was used to dichotomize the study group. We defined impaired glucose tolerance by 1) fasting glucose level of ≥140 mg/dl, 2) 1-hour glucose level of 200 mg/dl or higher, or 3) 2-hour glucose level of ≥150 mg/dl. Student’s t test determined whether the two groups differed in AIMS scores. For the following analyses, the AIMS total rating, including the severity assessment, was used as a continuous measure. Partial correlations indexed the association between glucose levels and movement ratings. Age was included in the partial correlation. Correlations were applied to fasting, 1-hour, and 2-hour serum measures. We also used partial correlations to associate insulin resistance with abnormal movements, controlling for concurrent glucose measures.
RESULTS
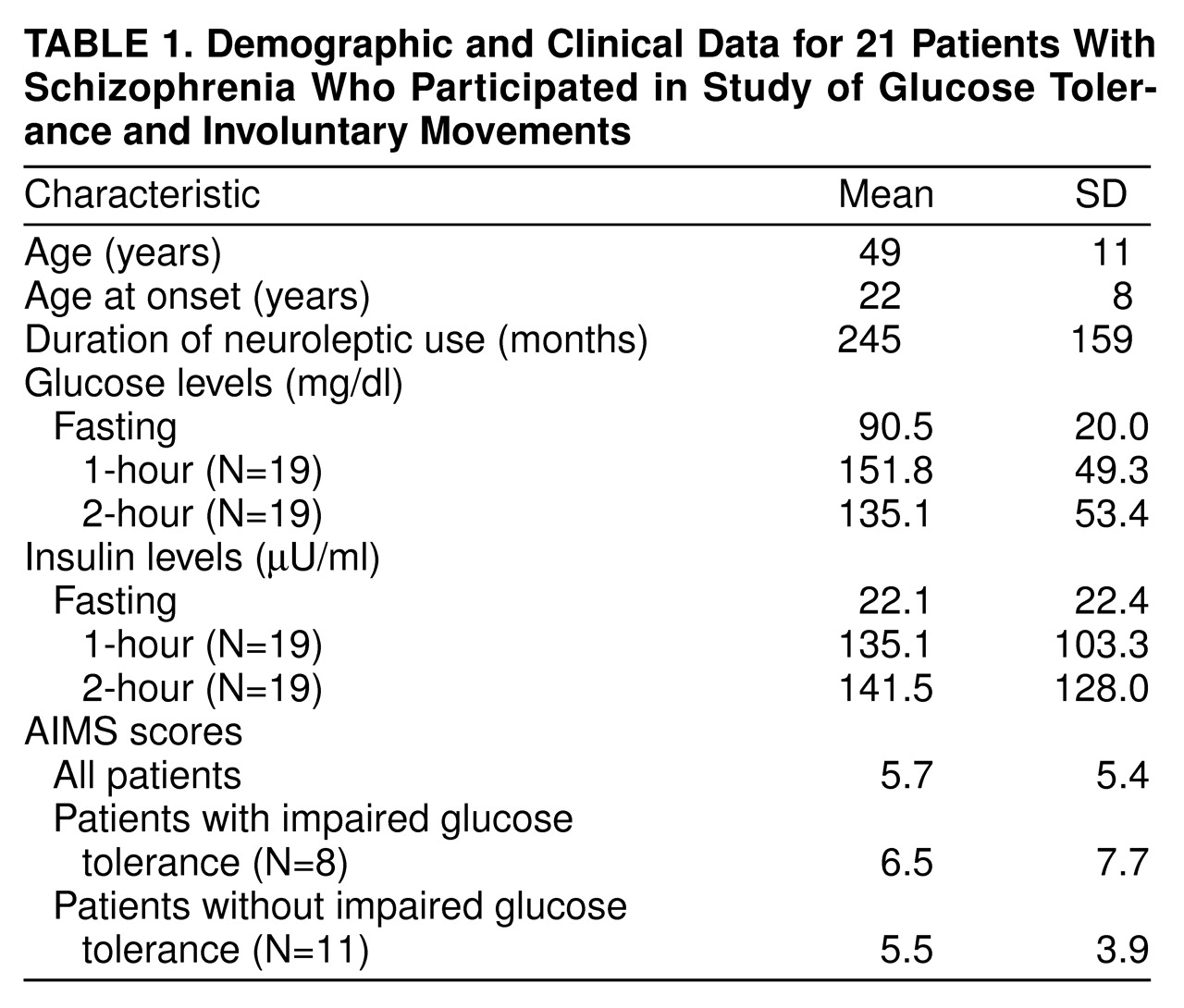
Fourteen of the patients were men, and seven were women. Findings from the analysis of other demographic information, as well as the AIMS scores, insulin values, and glucose values, are noted in
table 1. Nineteen subjects completed the full glucose tolerance test, and eight met criteria for impaired glucose tolerance. There was no significant difference between the impaired and normal glucose tolerance groups in AIMS scores (t=0.3, df=9.5, p>0.7 for unequal variances). We observed a significant correlation between AIMS ratings and baseline glucose measurements when we controlled for age (r=0.62, df=16, p<0.006). The correlation between fasting insulin levels and AIMS ratings, after we controlled for fasting glucose, was r=0.48 (df=16, p<0.04). The AIMS ratings distribution was skewed because a few individuals had severe movements, and the magnitude of the correlations was substantially influenced by these ratings. Correlations at 1 and 2 hours were nonsignificant.
DISCUSSION
These data suggest that a variety of factors, such as those mediating glucose control, may help explain the seemingly arbitrary occurrence of tardive dyskinesia. Although we did not demonstrate an association between overtly impaired glucose tolerance and abnormal movements, we did observe a possible relationship between abnormal movements and glucose levels in the fasting state. We also observed a relationship between abnormal movements and higher fasting insulin levels, which may reflect hyperinsulinemia associated with early impairment in glucose tolerance
(7).
The relationship between hyperglycemia and tardive dyskinesia is intriguing, lending itself to a number of proposed mechanisms. Animal studies suggest a mechanism of glucose-induced dopamine sensitivity, e.g., hyperdopaminergic activity in diabetic rats resolved with insulin treatment
(8). Along these lines, it has been speculated that low-dose insulin treatment may be of benefit in tardive dyskinesia. One study demonstrated dramatically decreased movements in 10 patients with schizophrenia and tardive dyskinesia given low-dose insulin injections over a period of 3 months
(9).
There are many layers of complexity among the interactions of dopamine, insulin, and glucose in the context of neuroleptic treatment. Gillman and Sandyk
(10) suggested that chronic neuroleptic administration is associated with increased activity of pancreatic insulin and increased fasting blood sugar. Thus, neuroleptics may confer a greater likelihood of insulin resistance. For example, Brambilla et al.
(11) reported that haloperidol therapy was associated with increased insulin release, and Mukherjee et al.
(12) observed that patients with tardive dyskinesia had greater hyperglycemic responses to glucose loading while receiving antipsychotic medication.
In addition to these studies, interesting observations were noted as early as 1945 suggesting that glucose metabolism is altered in schizophrenia as a function of the underlying disease process. For example, Franzen and Nilsson
(13) measured glucose and insulin values following insulin coma and reported that insulin resistance was greater in psychotic than nonpsychotic patients. Furthermore, Schimmelbusch et al.
(14) reported a positive correlation between insulin resistance and duration of hospitalization in unmedicated patients with schizophrenia.
In conclusion, diverse evidence has supported a possible disruption of glucose metabolism in schizophrenia, perhaps mediated by insulin resistance. The results reported here in this small study may reflect such a disruption, but they must be interpreted very cautiously and require validation through larger studies with richer samples that represent the spectrum of abnormal movement severity.