Currently, there is substantial research interest in hyperventilation, respiratory distress, and pulmonary physiology among patients with panic disorder. Enhanced sensitivity to CO
2 among patients with panic disorder was first reported by Gorman et al.
(1), which prompted research on producing panic attacks in the laboratory. Since then, numerous studies using different concentrations of CO
2 (5%, 7%, and 35%) repeatedly showed that patients with panic disorder experienced more panic attacks than both normal subjects and subjects with other psychiatric disorders
(2–
8). In addition, some studies suggested that CO
2 hypersensitivity might be related to a familial vulnerability to panic disorder and might be a disease-specific trait marker
(9–
11). Regarding the lactate and CO
2 literature and the clinical observation that patients with panic disorder often complain of dyspnea and hyperventilation, Klein
(12) proposed that panic attacks were related to the hypersensitivity of brainstem chemoreceptors. Such a dysfunction would make a person vulnerable to “false suffocation alarms,” namely, panic attacks. According to this hypothesis, CO
2 hypersensitivity is directly involved in the pathophysiology of panic disorder.
A wide range of physical and cognitive symptoms characterize the clinical picture of panic disorder with substantial variability in symptom profile, severity of symptoms, and phobic avoidance among panic patients. On the basis of clinical observations some reports have suggested that panic disorder could be divided into subtypes
(13–
16). Although these studies have described different symptom clusters as subtypes of panic disorder, all agree that a group of patients with “prominent respiratory symptoms” emerged as a distinct subtype. Briggs et al.
(15) further noted that the group of patients whose panic attacks are characterized by prominent respiratory symptoms suffered more spontaneous panic attacks and responded to imipramine, whereas patients with the nonrespiratory subtype suffered more situational panic attacks and responded more to alprazolam. These data suggest that there might be a subgroup of panic patients with prominent respiratory symptoms who are also more apt to experience panic attacks, according to the false suffocation alarm hypothesis.
We further investigated the subtyping of Briggs et al.
(15) by examining the CO
2 sensitivity rates of patients with the proposed panic disorder subtypes. Our hypothesis was that in patients with prominent respiratory symptoms, CO
2 challenge would precipitate panic attacks more easily; therefore, patients with the prominent respiratory subtype would show greater CO
2 sensitivity than patients with the nonrespiratory subtype.
RESULTS
Demographic and Baseline Variables
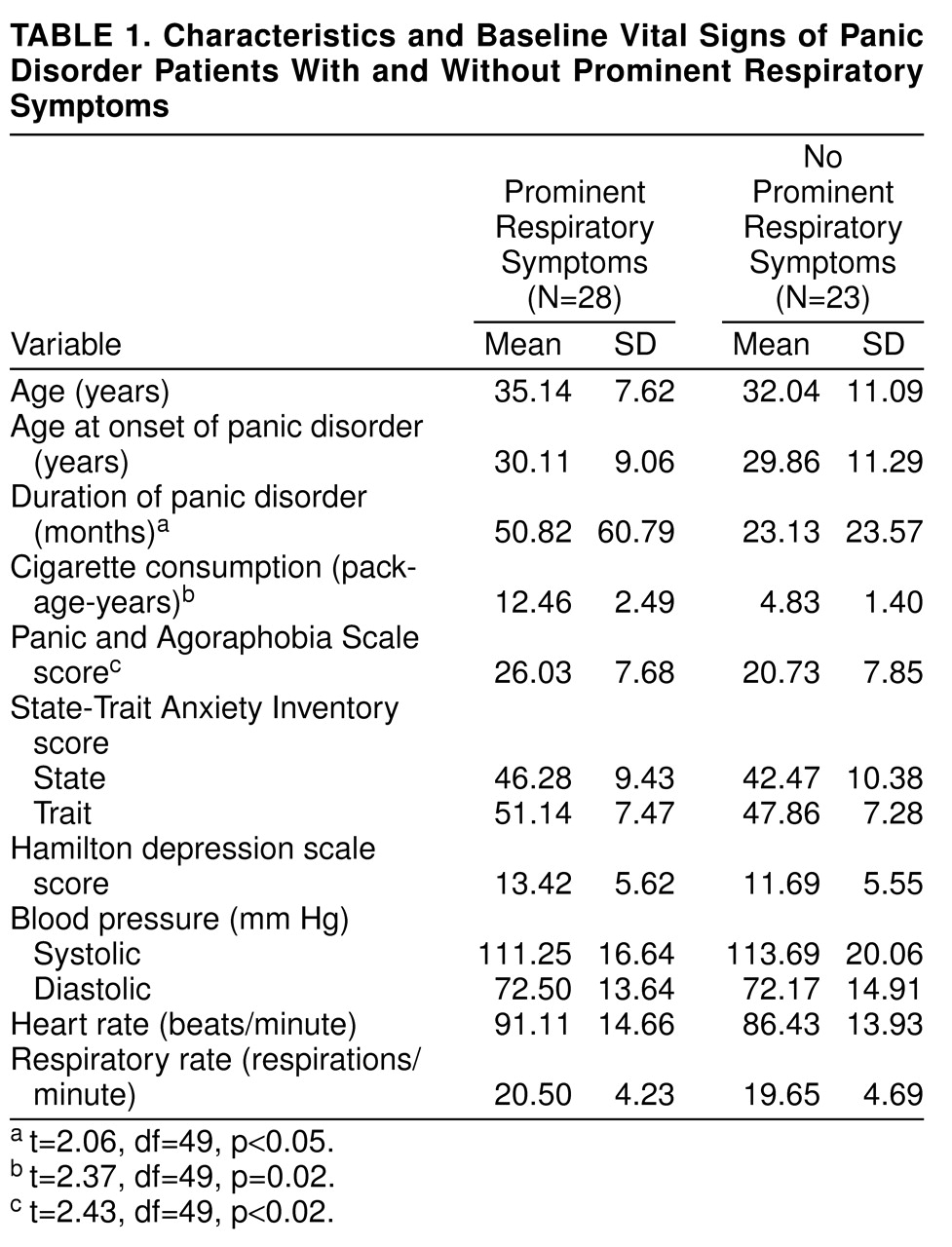
The mean age at onset of panic disorder was 30.0 years (SD=10.0). The mean duration of illness was 38.2 months (SD=50.2). There were 28 patients (19 women, nine men) in the prominent respiratory symptom subtype group and 23 (15 women, eight men) in the nonrespiratory symptom subtype group. Chi-square analyses among the two subtypes showed no significant differences in sex distribution, education, or marital and occupational status. According to t tests, there were no significant differences between the two subtypes in mean number of panic symptoms, mean age, and mean years of education.
Thirty-four patients (67%) met the DSM-III-R criteria for panic disorder with agoraphobia, whereas 17 patients (33%) met the criteria for panic disorder without agoraphobia. Concomitant SCID diagnoses were generalized anxiety disorder in six patients (12%), social phobia in one patient (2%), simple phobia in one patient (2%), and conversion disorder in one patient (2%). Patients with or without comorbid psychiatric diagnoses, patients subtyped as having panic disorder with or without agoraphobia, patients who did or did not panic in response to the CO
2 challenge, and patients in the respiratory or nonrespiratory subtype groups did not show any differences in the comparisons on the baseline mean values for pulmonary function tests and other baseline vital signs. Data on the baseline measurements of vital signs and some other clinical variables are presented in
table 1.
According to repeated measures ANOVA with the Bonferroni correction, two between-subjects effects emerged for heart rate measurements. There were significant interactions of smoking status (smokers versus nonsmokers) by subtypes (respiratory versus nonrespiratory) (F=4.53, df=1, 43, p=0.04) and subtypes by agoraphobia (with or without agoraphobia) (F=6.43, df=1, 43, p=0.02). No other between-subjects interactions were observed for any measurements.
Regarding within-subjects effects, a significant time effect (F=3.20, df=2, 86, p=0.05) and significant subtypes-by-time interaction (F=3.47, df=2, 86, p=0.04) for heart rate were found. The mean pre- and two postintervention measurements of heart rate were 91.11 (SD=14.66), 88.78 (SD=11.25), and 85.96 (SD=9.97) for the prominent respiratory subtype group and 86.43 (SD=13.93), 92.00 (SD=15.38), and 84.69 (SD=11.16) for the nonrespiratory subtype group, respectively, indicating that the heart rate of patients in the nonprominent respiratory subtype group was more responsive to CO2. In addition, baseline heart rate was higher in patients in the respiratory subtype group as a reflection of high level of baseline anxiety.
Time effect (F=6.41, df=2, 86, p=0.003) and agoraphobia-by-time interaction (F=3.84, df=2, 86, p=0.03) for systolic pressure were also significant. The systolic pressure after the first minute of challenge for patients without agoraphobia (mean=121.92 mm Hg, SD=21.68) was significantly higher than that for patients with agoraphobia (mean=113.20 mm Hg, SD=19.67). A significant time effect was found for respiratory rate (F=3.69, df=2, 86, p=0.03), indicating that the patients had a higher respiratory rate right after inhalation (mean=21.47 respirations/minute, SD=4.49) than they had before inhalation (mean=20.11 respirations/minute, SD=4.42).
Panic Rates
Thirty-three (65%) of 51 patients had a panic attack as a response to the CO2 procedure. One patient (2%) had a panic attack with limited symptoms, but we did not accept this response as a CO2-induced panic attack. Patients who experienced a CO2-induced panic attack had significantly higher baseline scores on the Panic and Agoraphobia Scale (mean=25.42, SD=8.34) than did patients without panic attacks (mean=20.38, SD=6.78) (t=–2.19, df=49, p<0.03). They also had significantly higher scores on the State-Trait Anxiety Inventory—State Form (mean=47.36, SD=10.13) than did patients without panic attacks (mean=39.44, SD=7.46) (t=–2.91, df=49, p<0.01). There were no statistically significant differences between panicking and nonpanicking patients on any other sociodemographic or clinical variables or on any baseline vital sign measurements. As expected, patients who had panicked in response to CO2 scored significantly higher on the Acute Panic Inventory (mean=10.57, SD=7.35) than did patients who had not panicked (mean=1.27, SD=1.44) (t=–5.29, df=49, p<0.001).
Stepwise logistic regression analysis revealed that higher baseline scores on the State-Trait Anxiety Inventory—State Form (Wald=5.76, df=1, p<0.02) and subtype status (Wald=5.03, df=1, p<0.05) predicted the CO2-induced panic response for the entire patient group.
Subtypes
Twenty-two (79%) of 28 patients in the prominent respiratory symptom subtype group and 11 (48%) of 23 patients in the nonrespiratory symptom subtype group experienced a panic attack. The panic rate in response to CO
2 among the panic disorder subtypes was significantly different (χ
2=3.97, df=1, p<0.05, with Yates’s correction). In addition, patients with prominent respiratory symptoms had significantly higher scores on the Panic and Agoraphobia Scale and had a longer duration of illness than patients with nonrespiratory symptoms (
table 1). Neither subtype differed significantly on any other baseline clinical variable.
In the entire study group, 37 patients (73%) had a history of smoking, and 14 patients (27%) reported that they had never smoked. The mean amount of cigarette consumption was 8.39 package-years (package of cigarettes consumed daily multiplied by years) (SD=8.92) for the study group. Regarding the lifetime smoking habits of patients in the two groups, for 21 patients (75%) in the prominent respiratory symptom subtype group and 16 (70%) in the nonrespiratory symptom subtype group, smoking preceded the onset of panic disorder. Cigarette consumption for patients in the prominent respiratory subtype group (mean=12.46 package-years, SD=2.49) appeared to be greater than that for patients in the nonrespiratory subtype group (mean=4.83 package-years, SD=1.40). The difference was significant (t=2.37, df=49, p=0.02). Patients in the prominent respiratory subtype group had a longer history of smoking (mean=150.21 months, SD=120.39) than patients in the other group (mean=89.74 months, SD=98.74); however, this difference did not reach statistical significance (t=1.93, df=49, p=0.06).
DISCUSSION
We found, as hypothesized, that patients with panic disorder with prominent respiratory symptoms were more sensitive to the CO
2 challenge than were patients with nonrespiratory symptoms. Our results are consistent with Klein’s false suffocation alarm hypothesis
(12). In a substantial group of panic patients, heightened CO
2 sensitivity may play an important role in the pathophysiology of panic disorder. Moreover, there may be a more specific association with prominent respiratory symptom subtype and CO
2 hypersensitivity.
Although implicating a biologically mediated pathophysiological process, treatment response data alone might be insufficient to create etiologically homogeneous patient subgroups. Both imipramine and alprazolam are beneficial in treating heterogeneous panic patient groups
(23–
25). It is therefore clear that additional biological markers are needed to establish a more precise subtyping. Although imipramine and alprazolam both seem to block CO
2-induced panic
(26–
29), CO
2 hypersensitivity can still be a valuable tool for delineating subtypes. In this study, only respiratory subtype and high baseline anxiety scores on the State-Trait Anxiety Inventory—State predicted a CO
2-induced panic attack. Taken together with the differential response to drugs, as reported by Briggs et al.
(15), our finding of differential CO
2 hypersensitivity of patients in these subtype groups strengthens the possibility of an accurate subtyping of panic disorder. A recent study
(30) reported that there are no response differences to either hyperventilation or 35% CO
2 challenges among medicated panic patients when they are classified on the basis of severity of dyspnea symptoms and low or high baseline partial CO
2 pressure levels. However, there is still far from complete agreement on the exact symptom profile of a respiratory subtype
(12–
16). In addition, an unusually low rate of panic response was observed in this study. Therefore, conflicting differences in results among studies may be attributable to consideration of merely dyspnea symptoms and disregard of some relevant panic symptoms for subtyping and the criteria used to define a panic response.
Our rather simple methods of assessing the baseline respiratory functions did not reveal any overt pathology or differences between panic disorder subtypes. However, it is still possible that abnormalities in respiratory physiology and disordered breathing in panic patients, as shown by others
(4,
31–
35), may yield differences between subtypes. Patients in the prominent respiratory subtype group have a significantly longer duration of illness, more severe panic and phobic symptoms, and greater disability, as reflected by higher scores on the Panic and Agoraphobia Scale, than patients in the nonrespiratory subtype group. The relevance of these findings to the subtype status was supported indirectly with treatment response studies, in which factors such as increased social disability and longer duration of illness predicted poorer response to alprazolam, whereas milder illness at baseline predicted a better response
(36,
37). Subtype and severity determinations of panic disorder in studies of treatment and respiratory psychophysiology will be more informative and should be considered in future research.
Furthermore, patients in the prominent respiratory subtype group are more likely to be heavier smokers than are patients in the nonrespiratory subtype group. Higher smoking prevalence in anxiety patients has been reported
(38). It is possible that panic patients with prominent respiratory symptoms may have a high level of anxiety that is reduced by smoking. Nevertheless, smoking itself could create the symptom of breathlessness and even dyspnea by inducing airway obstruction. Our finding of a high prevalence of smokers and a high amount of cigarette use among the panic patients, especially among those with prominent respiratory symptoms, suggests a possible predisposing agent for respiratory diseases, as well as for panic disorder. Although Verburg et al.
(39) studied only two respiratory panic symptoms in the determination of subtype, they reported a higher prevalence of bronchitis in panic patients with prominent respiratory symptoms. On the basis of other data on respiratory disease and panic disorder comorbidity
(40–
42), it is likely that respiratory diseases may predispose people to panic disorder and may account, at least in part, for the respiratory symptoms of panic disorder. However, without prospective studies relating smoking and bronchitis with panic disorder and subtypes, these suggestions will remain speculative.
A history of traumatic suffocation experiences and nocturnal panic attacks associated with the predominantly respiratory subtype have been recently reported
(43). Along with the literature and our data, observations suggest that it is worthwhile considering some noticeable clinical features that are helpful in distinguishing proposed subtypes. It appears that panic patients with prominent respiratory symptoms also have more spontaneous and nocturnal panic attacks, past traumatic suffocation experiences, past respiratory diseases, heavier smoking history, longer duration of illness, more sensitivity to CO
2 challenge, and more responsiveness to tricyclic treatment and that this subtype might be related to a more severe and disabling type of panic disorder
(15,
39,
43).
A number of methodological limitations of this study deserve mention. First, focusing on only two subtypes of panic disorder was the major limitation of this study. There are studies implicating other subtypes such as vestibular, gastrointestinal, cardiac, and mixed
(13–
16,
44,
45), in which research on CO
2-induced panic is relevant. Second, baseline arterial blood gas measures, tidal volume,
and end-tidal CO
2 pressure measurements were not available in the present study. These measurements may be important for identifying the respiratory abnormalities that could also predict the subtype differences. Third, an unknown bias might have prevailed because the researchers were not blind to the panic disorder diagnosis. Despite these shortcomings, we believe that our findings still have convincing implications in panic research.
In conclusion, differential responses to CO2 challenge have provided a stronger biological basis to a preliminary subtyping. This underscores the need for further biological and phenomenological research to determine the exact symptom profile of the subtypes. Precise subtyping of panic patients in turn, by lowering diagnostic heterogeneity, will reduce most of the contradictory findings across the panic disorder studies.