Subjects
The subjects in this investigation were pooled from two clinical trials, each with three parallel treatment arms and identical designs: a double-blind trial of oral progesterone, alprazolam, and placebo for treatment of severe PMS in 185 subjects
(6) and a comparison of serotonergic and noradrenergic antidepressants for treatment of PMS in 145 subjects
(7). In both studies, significant placebo-drug differences in treatment response were demonstrated. In the first study, alprazolam was significantly better than placebo or progesterone
(6). In the second study, sertraline was significantly better than placebo or desipramine
(7).
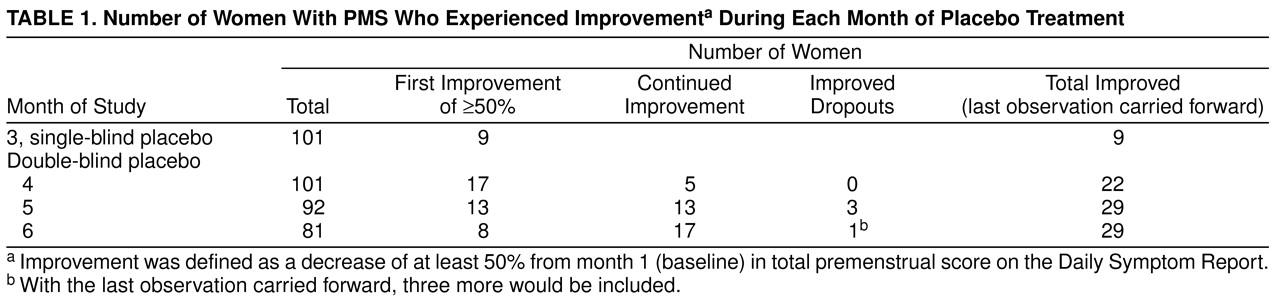
The subjects in both studies were unimproved according to the study criteria after single-blind placebo medication and were randomly assigned to 3 months of double-blind treatment. Of 514 subjects who entered the single-blind placebo cycle before random assignment in the two studies, 35% discontinued treatment in the single-blind phase—18% because of improvement as reported by the subjects or as defined by the study criteria and 17% for other reasons. In this report of subjects randomly assigned to the placebo arms (N=101), 55 subjects were from study 1 and 46 subjects were from study 2.
Before the two placebo groups were combined, the mean premenstrual scores on the Daily Symptom Report
(8) were compared, and they showed no significant differences in any of the 6 study months. At month 1 the mean scores were 138 (SD=75) for study 1 (N=55)
(6) and 155 (SD=77) for study 2 (N=46) (unpublished data) (t=1.15, df=99, p=0.25). At month 6 the mean Daily Symptom Report scores were 111 (SD=58) and 118 (SD=94), respectively (t=0.35, df=79, p=0.73). Demographic background variables did not differ between the two study groups.
The study designs for the two groups were identical and consisted of six menstrual cycles in four study phases.
1. Prescreening—one cycle of daily symptom ratings before the first clinical visit.
2. Screening—one cycle of evaluation with no medications and two clinical visits, one postmenstrual and one premenstrual.
3. Placebo lead-in—one cycle of single-blind administration of placebo capsules.
4. Randomized, double-blind treatment—three cycles of placebo capsules.
The studies were approved by the Institutional Review Board of the University of Pennsylvania. After complete description of the study to the subjects, written informed consent was obtained.
All subjects were 18–45 years of age, had regular menstrual cycles of 22 to 35 days, had experienced PMS for at least 1 year, reported moderate to severe interference with work, relationships, or social activities, and were in overall good health as determined by medical history, physical examination, laboratory tests of blood count, and complete blood chemistry with differential. The subjects had no current major psychiatric illness as determined by the Structured Clinical Interview for DSM-IV (SCID)
(9) or the SCID for DSM-III-R
(10), used for the earlier study group. According to the SCID interviews, 48% of the subjects met the DSM criteria for one or more previous illnesses: major depression (40%), alcohol or drug abuse or dependency (17%), eating disorders (3%), panic disorder (3%), manic syndrome (3%), or dysthymia (1%). The subjects took no concomitant treatments for PMS during the study.
The PMS criteria for study eligibility were a premenstrual total score of 70 or higher on the Daily Symptom Report with an increase of 50% or more from the postmenstrual total score, premenstrual symptom interference with functioning at a moderate to severe level as rated by the subjects, no current diagnosis of a major mental disorder as determined by SCID interview
(9,
10), and confirmation of symptom status by daily ratings in the 3-month screening period. Subjects who did not continue to meet these criteria after the single-blind placebo month were not assigned to double-blind treatment. In addition to meeting these criteria, 71% of the subjects met the DSM-IV criteria for premenstrual dysphoric disorder. Those who did not meet those criteria had a mean number of 2.3 qualifying symptoms of premenstrual dysphoric disorder, rather than the required five symptoms.