Antipsychotic medications are known to alter the structure and metabolism of basal ganglia in humans and animals
(1–
7). In patients with schizophrenia, typical antipsychotics induce striatal enlargement, particularly in the caudate, putamen, and globus pallidus
(7–
9). In contrast, the majority of studies of patients switched from typical antipsychotics to clozapine report decreases in caudate volume
(6,
10–13). The effects in humans of alternate atypical antipsychotics (risperidone, olanzapine, quetiapine, ziprasidone) are less understood. In one published study, long-term administration of risperidone did not induce striatal enlargement
(7). The effects seen in animal studies are inconsistent. In rats, both increases and decreases in striatal volumes occur after administration of either haloperidol or clozapine
(14,
15). In comparison, long-term administration of olanzapine in rats decreases striatal volumes
(15).
The effects of antipsychotic medications on basal ganglia metabolism may vary depending on medication type and patient characteristics
(16,
17). Haloperidol does not reduce striatal glucose metabolism and differentially reduces cortical glucose metabolism, with nonresponsive patients experiencing decreases and responsive patients experiencing no changes in metabolic activity
(18). In contrast, a study of clozapine and fluphenazine showed that both medications decreased striatal and cingulate glucose metabolism, with female subjects experiencing a greater change in glucose utilization than male subjects
(19). Risperidone was also reported to decrease striatal glucose metabolism after 6 weeks of treatment
(20). Parallel rat studies showed that haloperidol has little effect on neuronal activity levels in the striatum, whereas risperidone and clozapine decrease activity in the substantia nigra reticulata nucleus of the basal ganglia system in a dose-dependent manner, thus differentially affecting the output of the basal ganglia system
(21). Despite their potential for differential effects, few studies have made direct drug-to-drug comparisons.
We performed two separate studies to examine in chronically treated schizophrenia patients the effects on striatal volumes of switching from treatment with either typical antipsychotics or risperidone to olanzapine. In the first study, patients were switched from typical antipsychotic medications to olanzapine and compared with healthy volunteers. Caudate, putamen, and pallidal volumes were expected to decrease after switching from typical antipsychotics to olanzapine. The severity of extrapyramidal symptoms was also expected to decrease after switching. In the second study, all subjects were taking risperidone at baseline. Subsequently, a subgroup was switched to olanzapine. The decision to switch treatments was based on clinical evaluation of the patients’ overall clinical response to risperidone. Olanzapine is pharmacologically more similar to clozapine than risperidone
(22), suggesting that the effects of olanzapine on basal ganglia volumes may be similar to those of clozapine
(10,
13,
23). Additionally, olanzapine is less likely to induce extrapyramidal symptoms compared with risperidone at comparable doses, as is clozapine
(24–
26). In the second study, basal ganglia volumes were expected to decrease after switching from risperidone to olanzapine, as were extrapyramidal symptoms.
Method
Subjects
Thirty-seven patients with DSM-IV schizophrenia and 23 healthy comparison subjects were included in this study. No subjects in this study received adjunct treatments for mood disorders during the course of treatment. The baseline scans of 15 patients were included in a previous study, and both baseline and outcome scans were reported previously for 17 of the healthy comparison subjects
(7). Subjects were recruited through the Nova Scotia Early Episode Psychosis Program in Halifax. Approval was obtained from the Dalhousie University Ethics Committee. Informed written consent was obtained from all subjects. Exclusion criteria were a history of significant head injury or loss of consciousness exceeding 5 minutes, a history of facial or nasal trauma, a history of DSM-IV substance abuse, a current diagnosis of substance abuse during treatment or at follow-up, a history of seizure disorder, or a family history of psychotic disorders. Patients were reassessed after a mean interval of 45.6 weeks.
Treatment and Clinical Measures
Clinical assessments included the Positive and Negative Syndrome Scale
(27) and the Extrapyramidal Symptom Rating Scale, a comprehensive rating of extrapyramidal symptoms and signs
(28). Global scores on the Extrapyramidal Symptom Rating Scale subscales are reported. Interrater reliability for clinical measures based on intraclass correlations (ICCs) were high (Positive and Negative Syndrome Scale: ICC=0.85; Extrapyramidal Symptom Rating Scale: ICC=0.89). All ratings were performed by trained clinicians (L.C.K. and Heather M. Milliken, M.D., F.R.C.P.C.).
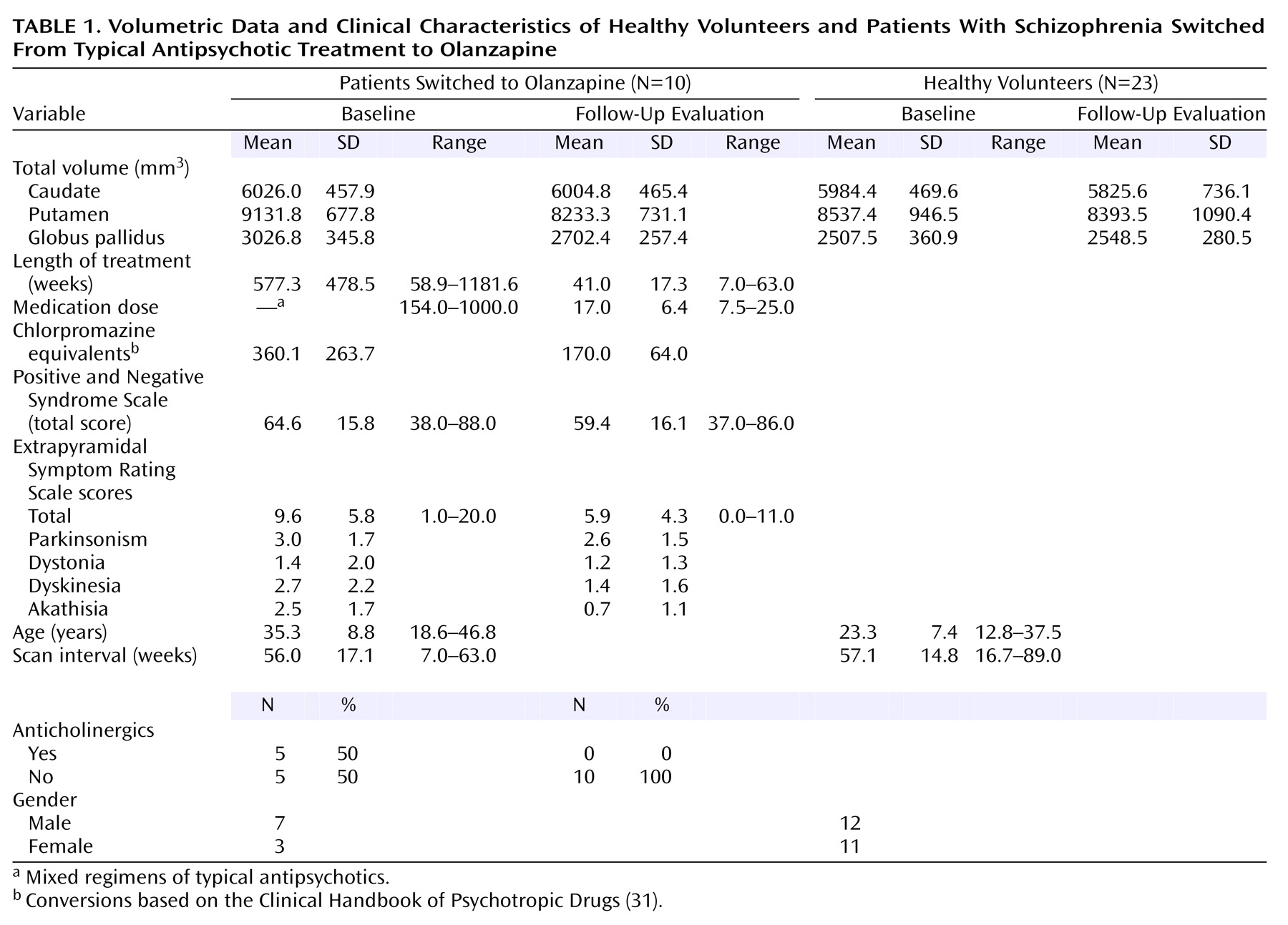
The first study investigated the effects of switching from typical antipsychotics (loxapine, trifluoperazine, chlorpromazine, fluphenazine, haloperidol) to olanzapine. Some patients were receiving additional anticholinergic medications to ameliorate extrapyramidal symptoms at baseline (
Table 1). No patients required anticholinergic agents at follow-up.
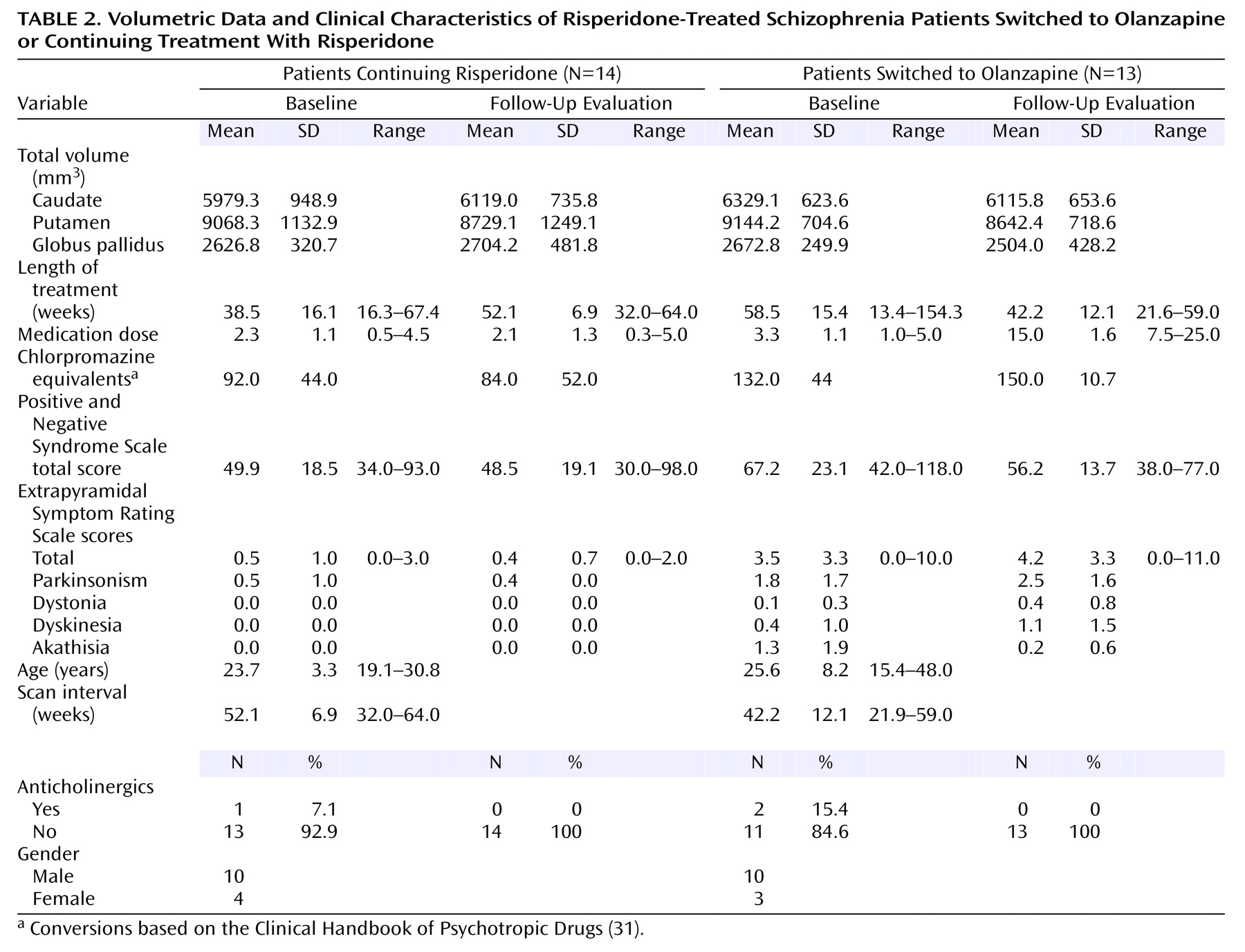
Both patient groups in the second study were being treated with low to moderate doses of risperidone at baseline (
Table 2). Thirteen patients were switched to olanzapine treatment, and 14 continued receiving risperidone. Medications were switched on the basis of a clinical evaluation by a psychiatrist. Those patients switched from risperidone to olanzapine exhibited more severe extrapyramidal symptoms and more severe psychiatric symptoms at baseline compared with those who continued risperidone treatment (
Table 2). Complete follow-up clinical assessments were available for 10 of the 14 patients who continued risperidone and 11 of the 13 patients switched to olanzapine.
No patients in either study required mood stabilizing or antidepressant medications.
Scanning and Measurement Protocols
Subjects were scanned with a Siemens Magnetom Vision 1.5-Tesla MRI scanner. An inversion recovery sequence in the coronal plane was obtained. The inversion recovery sequence was obtained as follows: TR/TE=2000/20 msec, field of view=200 mm, matrix=168×256 pixels. A total of 18 slices, 4 mm thick with a 1-mm interslice gap, were available for this sequence. Inversion recovery images were chosen for their superior white-gray tissue contrast. The white-to-gray pixel intensity for the images obtained with the inversion recovery sequence was 1.42, which compared favorably with three-dimensional volumetric spoiled gradient recall acquisition (pulse sequence) data from the same scanner that had a pixel intensity ratio of only 0.89. A trained rater made manual selections for all regions of interest using interactive shareware (NIH Image 1.62 pcc)
(29). Selections were made based on Duvernoy’s atlas of the human brain
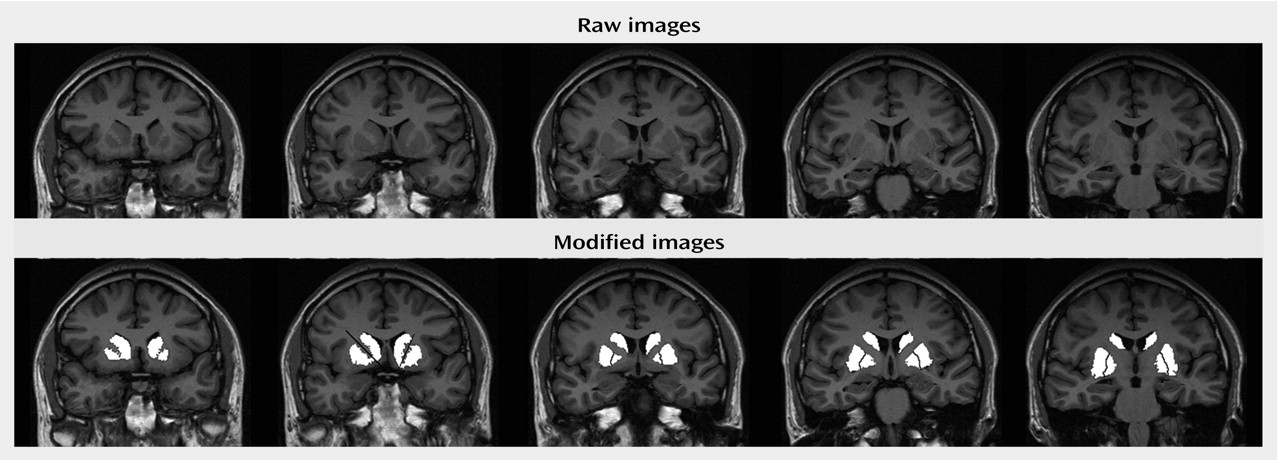
(30). Measurements began two slices anterior to and ended two slices posterior to the anterior commissure slice to cover a total distance of 25 mm in the anterior commissure-posterior commissure plane (
Figure 1). Anatomically, this protocol excluded the anterior-most portion of the head of the caudate (5-mm depth), the tail of the caudate, and the posterior-most putamen. Similarly, the posterior-most globus pallidus was excluded. The rater was blind to diagnosis, treatment, gender, and time of scan. All measures were repeated four times. Final volumes were calculated on the mean of four repetitions to reduce the possibility of rater error. Volumes were calculated on absolute slice thickness across all five slices. Total brain volumes were assessed from axial slices. Axial slices were obtained with a T
2-weighted sequence; TR/TE=4000/90 msec, field of view=220 mm, and matrix=238×256 pixels. Slice thickness for all T
2-weighted images was 5 mm with a 1-mm interslice gap, 22 slices were obtained in each T
2-weighted plane. Digitized slices were measured using a Macintosh G4 PowerMac computer. All scans were reviewed by a neuroradiologist (J.S.L.). Intrarater reliability for all regions was greater than 0.90 (caudate: ICC=0.98; putamen: ICC=0.96; globus pallidus: ICC=0.97; total intracranial volume: ICC=0.99).
Data Analysis: Statistical Methods
Initial comparisons of left and right striatal volumes did not reveal any significant left-right asymmetries, therefore subsequent analyses were based on total (left plus right) volumes. An initial ANOVA of total brain volume did not reveal any differences between groups. Comparisons of the effects of treatment over time were made with a repeated measures analysis of variance, with time (baseline, follow-up) and region (caudate, putamen, globus pallidus) as within-subject factors, and group (schizophrenia patients, healthy subjects [first study]; continued with risperidone, switched to olanzapine [second study]) as a between-subject factor. Multivariate analyses of covariance (MANCOVAs) were performed to compare basal ganglia volumes between groups at baseline or at follow-up, using the factor group, the covariates intracranial volume and age at time of scan, and the dependent measures caudate, putamen, and globus pallidus volumes.
For analysis of changes in extrapyramidal symptoms, paired t tests were used with the Extrapyramidal Symptom Rating Scale data, with Bonferroni alpha set at p=0.01 to control for five comparisons (total score, parkinsonism, dyskinesia, dystonia, and akathisia). Additional descriptive statistics of anticholinergic usage at follow-up were tabulated.
Results
Schizophrenia Patients Versus Healthy Comparison Subjects
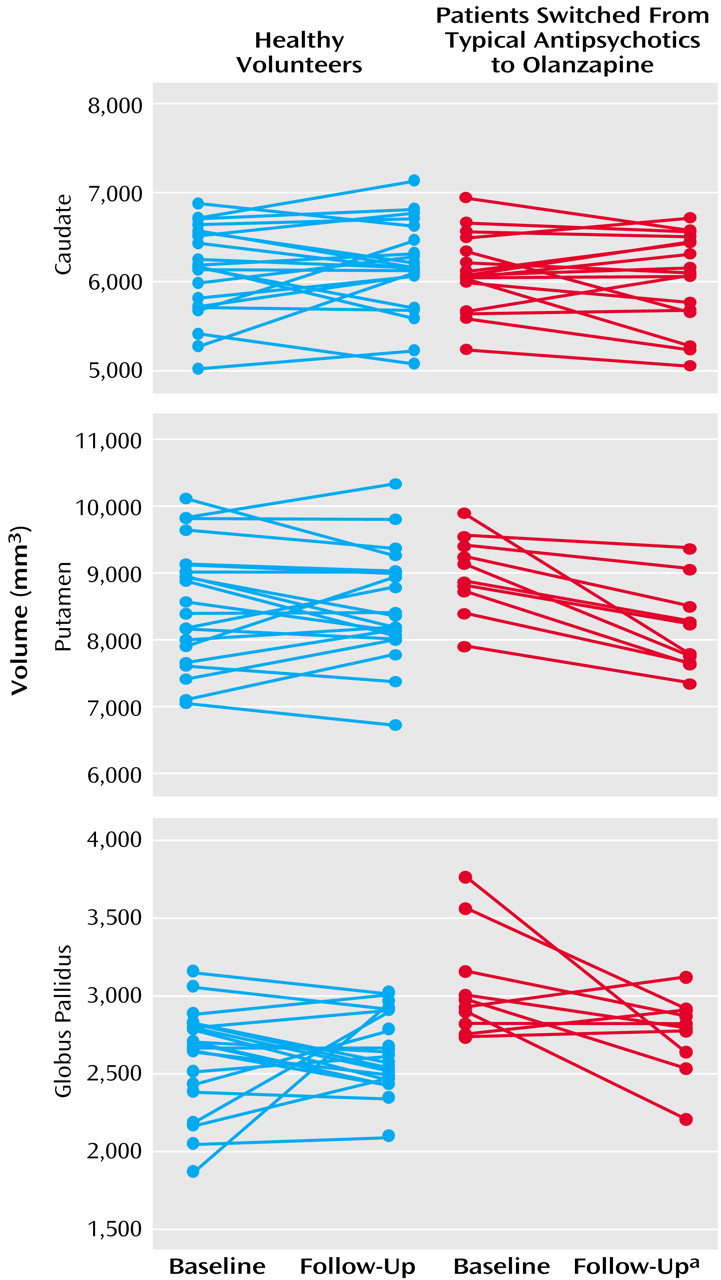
At baseline, patients treated with typical antipsychotic drugs had overall larger basal ganglia structures than healthy comparison subjects (Wilks’s lambda F=7.68, df=3, 27, p=0.0007). Differences were statistically significant for the putamen (7.0% larger, F=9.11, df=1, 29, p=0.005) and the globus pallidus (20.7% larger, F=24.06, df=1, 29, p=0.0001). For analysis of changes over time in patients after medication switch relative to comparison subjects, the MANCOVA indicated statistically significant effects of group (Wilks’s lambda F=5.05, df=3, 29, p=0.006), time (F=4.82, df=3, 29, p=0.008), and a group-by-time interaction (F=5.61, df=3, 29, p=0.004). As seen in
Figure 2, basal ganglia volumes decreased over time in the patients switched from typical antipsychotics to olanzapine, while volumes remained steady in healthy comparison subjects. Subsequent analyses indicated volume decreases in the putamen (smaller by 9.8%) and globus pallidus (smaller by 10.7%) associated with change from typical antipsychotics to olanzapine. At follow-up, there were no statistically significant differences in basal ganglia volumes between patients and healthy comparison subjects.
At baseline, five of 10 patients being treated with typical antipsychotic agents were receiving adjunct anticholinergic medications (
Table 1). While mean Extrapyramidal Symptom Rating Scale scores decreased following the switch to olanzapine, this was not statistically significant (t=1.82, df=9, p>0.10). However, at follow-up none of the 10 patients in this group were being treated with anticholinergic medications. Examination of individual Extrapyramidal Symptom Rating Scale subscores revealed a statistically significant decrease in akathisia scores (t=3.59, df=19, p=0.007) but not in parkinsonism, dystonia, or dyskinesia.
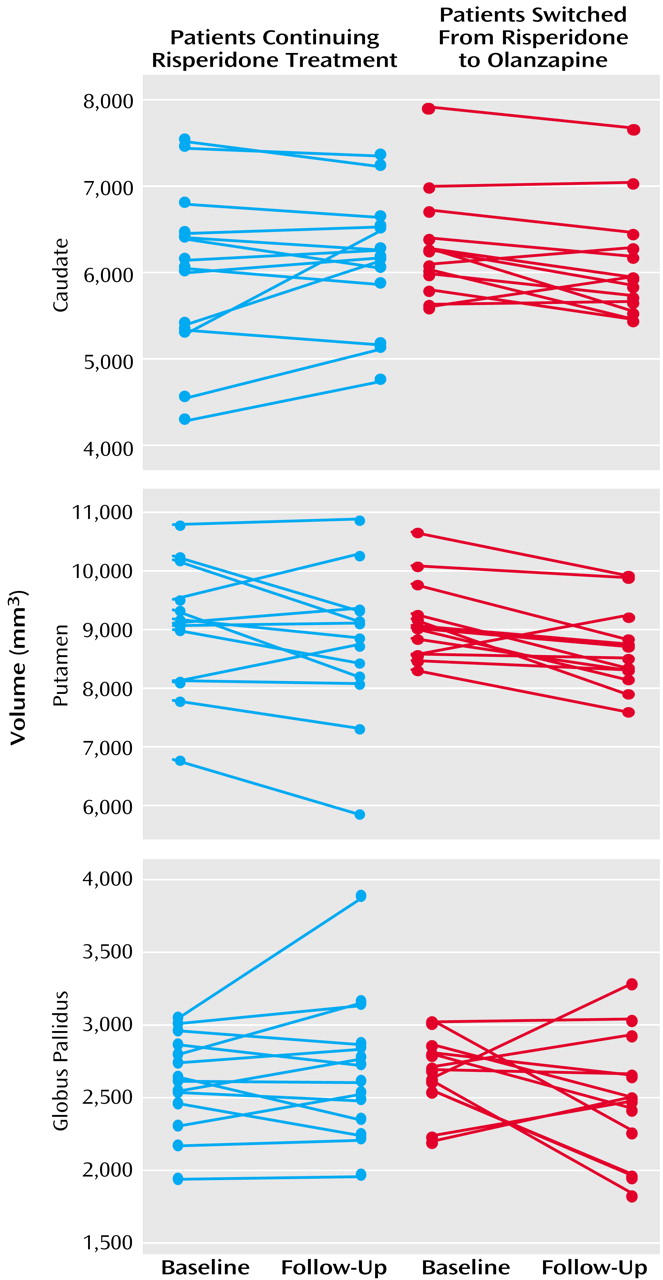
Risperidone Continuation Versus Switch to Olanzapine
As seen in
Figure 3, basal ganglia volumes of risperidone-treated patients subsequently switched to olanzapine did not differ at baseline from those continuing treatment with risperidone (Wilks’s lambda F=0.55, df=3, 21, p=0.65). For analysis of changes over time, the MANCOVA indicated a significant effect of time (F=4.41, df=3, 23, p<0.02) but no statistically significant effects of group (Wilks’s lambda F=0.25, df=3, 23, p=0.86) or group-by-time interaction (F=2.87, df=3, 23, p=0.059). At follow-up, no statistically significant differences between the groups in overall basal ganglia volumes were observed (Wilks’s lambda F=1.04, df=3, 21, p=0.39).
Mean total Extrapyramidal Symptom Rating Scale scores at baseline in patients switched to olanzapine were higher than scores in patients continuing risperidone treatment (t=2.85, df=21, p=0.01) (
Table 2). Extrapyramidal Symptom Rating Scale total scores at the follow-up evaluation did not significantly differ from baseline scores for either the patients continuing risperidone treatment (t=–1.00, df=9, p>0.34) or those switched to olanzapine (t=–0.37, df=10, p>0.70). Examination of the subscales of the Extrapyramidal Symptom Rating Scale did not reveal significant changes in any scores.
Discussion
As expected, treatment with typical antipsychotics was associated with larger basal ganglia volumes, and switching to olanzapine was associated with reduction in basal ganglia volumes. Specifically, the putamen and globus pallidus volumes were normalized following the switch to olanzapine. The pattern of regional changes differs somewhat from two earlier reports of the effects of switching from typical antipsychotic medications to clozapine. Following switching, Chakos et al.
(3) reported a reduction in caudate volume in adult patients, and Frazier et al.
(12) found reduction in both the caudate and the globus pallidus in childhood-onset schizophrenia patients. The regional inconsistencies may be a reflection of specific effects of previously administered typical antipsychotics or differences in effects of clozapine and olanzapine. The role of the putamen in both the presentation of schizophrenia-related symptoms and extrapyramidal symptoms is not fully understood. An earlier study by Stratta and colleagues
(32) demonstrated a significant correlation of performance on the Wisconsin Card Sorting Test and left-sided putamen volume, suggesting a role in executive functioning, which is known to be affected in schizophrenia
(33,
34).
Eight out of 10 patients receiving typical medications at baseline had movement disorders according to the Extrapyramidal Symptom Rating Scale. This scale covers a full range of potential motor abnormalities and is sensitive to subtle movement disorders
(35). The mean total baseline Extrapyramidal Symptom Rating Scale scores were likely partially ameliorated by anticholinergic medication. These are most effective in treating tremors, rigidity, and bradykinesia but have low efficacy for treating antipsychotic-induced akathisia
(36). The observed reduction in akathisia after the switch to olanzapine may be a true reflection of olanzapine’s low propensity to induce extrapyramidal symptoms.
In our second study, overall basal ganglia volumes did not differ between patients with good and poor responses to risperidone. Subsequent switching to olanzapine in those with a poor response to risperidone was not associated with a significant change in basal ganglia volume. This observation suggests the effects of olanzapine on basal ganglia volume in patients previously treated with typical antipsychotics represent normalization rather than atrophy.
Neither overall symptom severity nor overall extrapyramidal symptom severity changed when patients with a poor response to risperidone were switched to olanzapine. However, patients previously receiving typical antipsychotics did have moderate reductions in dyskinesia and akathisia. The extrapyramidal symptoms present in the group of patients poorly responsive to risperidone may be related to a different mechanism, perhaps intrinsic to schizophrenia, that is less responsive to switching to olanzapine than are extrapyramidal symptoms related to typical antipsychotic medications. Moreover, relative dosing with respect to D
2 affinity before and after switching from risperidone to olanzapine remained relatively equal, thus there would be little expectation for a change in extrapyramidal symptom severity
(37). The relationships among antipsychotic dose, striatal volume, and extrapyramidal symptom severity are not clear. Data from this study do not demonstrate any relationship of either total dose or current dose of antipsychotic medication being correlated with striatal volumes, change in striatal volumes, or severity of extrapyramidal symptoms scores (all exploratory regression analyses had r values <0.40 and corresponding p values >0.05). This suggests that striatal volume, while responsive to specific types of antipsychotic agents, does not affect the presence or severity of extrapyramidal symptoms.
Summary
The main findings of the present study were significant reductions in putamen and globus pallidus volumes in patients switched to olanzapine from typical antipsychotics. These subregional-specific results may be due to differential effects of olanzapine on striatal structures or of an unknown sampling bias of the subjects chosen for this study. As well, the results from the risperidone-to-olanzapine group may only be valid for those patients who have partial or poor risperidone response. Additional variance in the volumetric measures may have been related to MRI slice thickness and slice angulation. However, a comparison of volumes reported in other studies with thinner slices did not reveal any deviation in the volumes reported in this current study
(5,
38).
Individual atypical antipsychotic agents exhibit specific neurochemical pathways of activity, and this specificity may contribute to differential volumetric changes in the striatum in response to antipsychotic challenge
(15,
39). In a study of striatal volumes in rats, caudate or putamen volumes were significantly increased by chronic exposure to haloperidol and clozapine
(15). In contrast, this same study found that chronic exposure to risperidone had no effect on caudate or putamen volumes whereas long-term exposure to olanzapine resulted in a significant decrease in striatal volumes
(15). Additionally, a recent study employing fMRI in patients with schizophrenia demonstrated differential activation of the caudate and putamen during a cognitive challenge
(40). Signal intensity was reduced in the putamen and anterior cingulate during testing in patients relative to healthy subjects, but not in the caudate. These findings are supported by differential responses to individual antipsychotic medications
(41). These differences are likely related to different receptor-targeting profiles of specific antipsychotic agents
(39). While the evidence for increased striatal volumes in humans or animals exposed to typical antipsychotic medications treatment is convincing
(1,
3,
4,
6,
7,
10–
12,
14,
15,
42), there are no data suggesting that clozapine increases striatal volumes. The majority of published reports in human subjects indicated that exposure to clozapine is associated with a reduction in striatal volumes in patients previously exposed to typical antipsychotics
(12–
14,
23). Studies of risperidone’s and olanzapine’s effects on striatal volumes after chronic administration are far fewer in number
(7,
15). The findings from the current study in conjunction with those from animal studies suggest that closer examination in subpopulations of patients with schizophrenia is required to clarify the true physiological and clinical effects of individual antipsychotic medications in the treatment of schizophrenia.