Schizophrenia is characterized by positive symptoms (such as hallucinations and delusions), negative symptoms (such as blunt affect), cognitive impairment, and affective symptoms
(1) . Cognitive functions involving working memory—the ability to hold and manipulate pieces of information online for subsequent use in planning and acting—are particularly impaired in schizophrenia
(2) . Working memory is critically dependent on prefrontal cortical functioning
(3) . Patients suffering from schizophrenia show aberrant activation of the prefrontal cortex during working memory tasks, which suggests a role for the prefrontal cortex in the pathophysiology of cognitive deficits in schizophrenia
(4) . Animal and human studies have highlighted the significance of dopamine in regulating prefrontal cortical activity related to cognitive processing
(5) . Accordingly, the dopamine hypothesis of schizophrenia suggests that reduced dopamine function in the prefrontal cortex is responsible for the cognitive deficits encountered in patients with the disorder
(6,
7) .
At the receptor level, D
1 receptor function is essential for working memory function
(8) . It has been suggested that D
1 receptors in the prefrontal cortex, located mostly in apical dendrites and spines of pyramidal neurons
(9), modulate the postsynaptic neuron’s response to incoming depolarizing, mostly glutamatergic, signaling
(8) . This modulation seems to be dependent on the state of the target neurons, and it seems to protect task-associated working memory representations from interfering and distracting stimuli by tuning the functional state of the target neurons
(10) . Too much or too little stimulation at the D
1 receptor can impair working memory, which suggests that the dependence of working memory on D
1 receptor stimulation may be described by an inverted U-shaped curve
(11) .
Schizophrenia is highly heritable
(12), but the molecular basis of its heritability has remained largely unknown. The search for quantifiable mediators of this genetic vulnerability (intermediate phenotypes, or endophenotypes) could facilitate the exploration of the exact pathophysiological mechanisms behind the genetic vulnerability for schizophrenia
(13) . Recent evidence suggests that neuropsychological deficits
(14), reduction of prefrontal cortical gray matter
(15), and prefrontal cortical dopaminergic dysfunction
(16) are associated with an increased genetic risk of schizophrenia. We recently demonstrated an increased density of dopamine D
2 receptors in the caudate nuclei of unaffected identical co-twins of patients with schizophrenia
(17) that was similar in magnitude to the overall increase in density observed in a meta-analysis of studies of patients with schizophrenia
(7) . However, as yet, the role of D
1 receptors in the genetic etiology of schizophrenia has remained unaddressed.
The aim of the study was twofold: to investigate the effects of genetic liability to schizophrenia on D 1 receptor binding by comparing unaffected co-twins of patients with schizophrenia with matched healthy comparison twin pairs, and to examine disease- and treatment-specific contributions by comparing medicated patients with their own unaffected co-twins.
Results
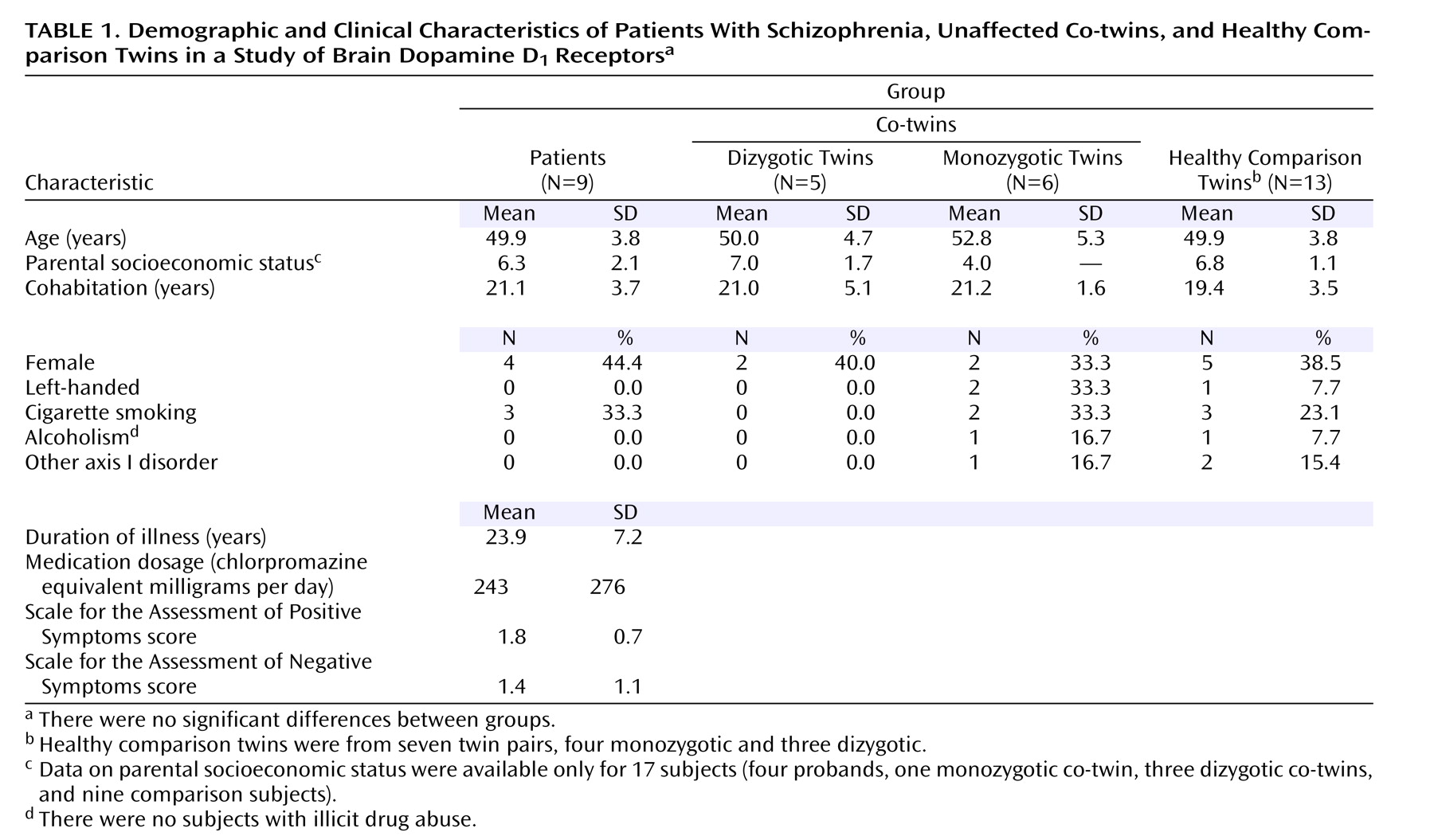
Table 1 summarizes the demographic and clinical characteristics of the study groups, and
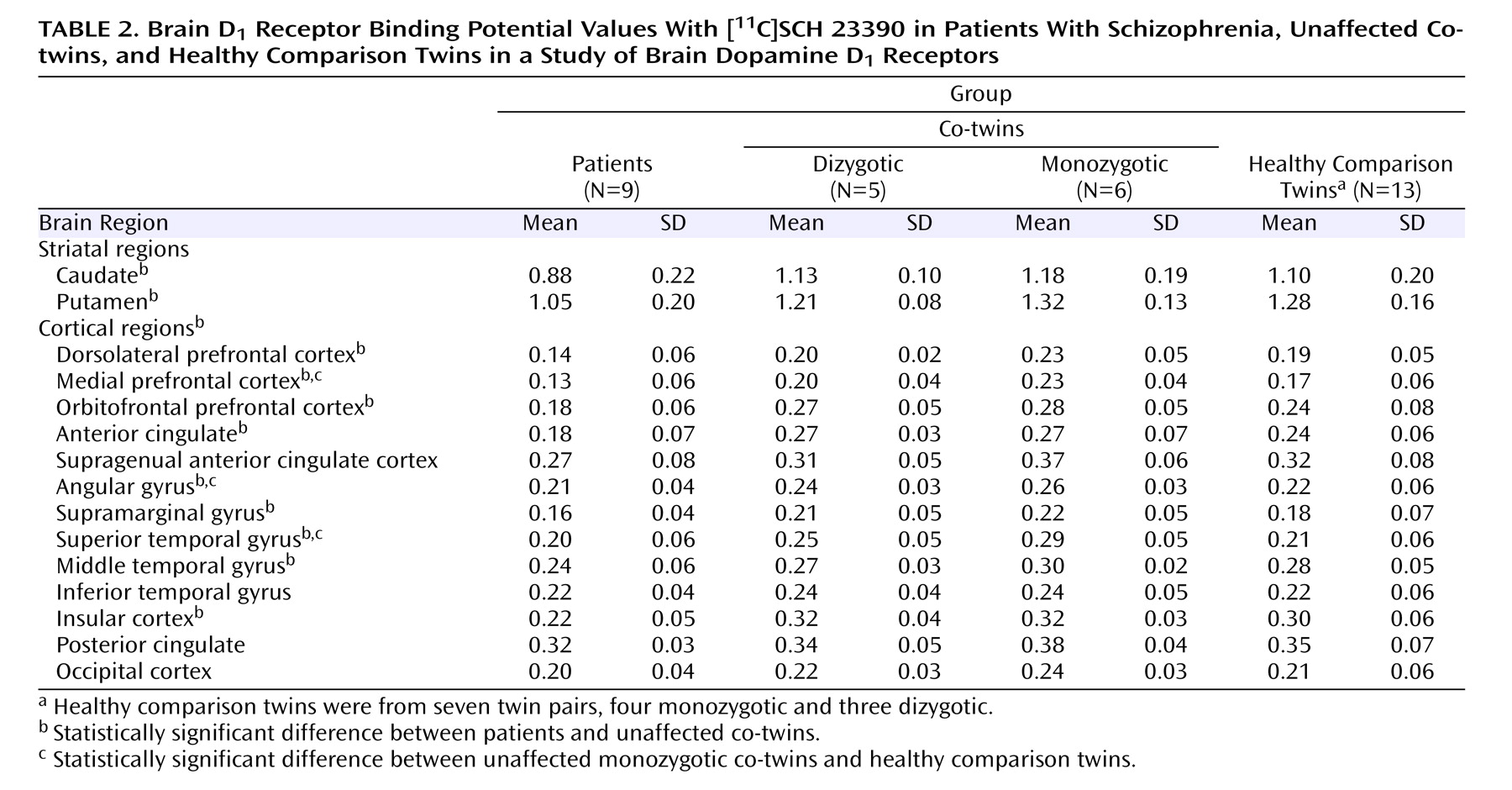
Table 2 lists the [
11 C]SCH 23390 binding potential values for each group. Significant group effects were found in various brain regions: the anterior cingulate cortex, angular gyrus, caudate, dorsolateral prefrontal cortex, medial temporal gyrus, superior temporal gyrus, insular cortex, medial frontal cortex, orbitofrontal cortex, putamen, and supramarginal gyrus (all p<0.0033; statistics available on request). We then looked at individual brain regions to see if unaffected co-twins differed from healthy comparison twins. Monozygotic co-twins had higher D
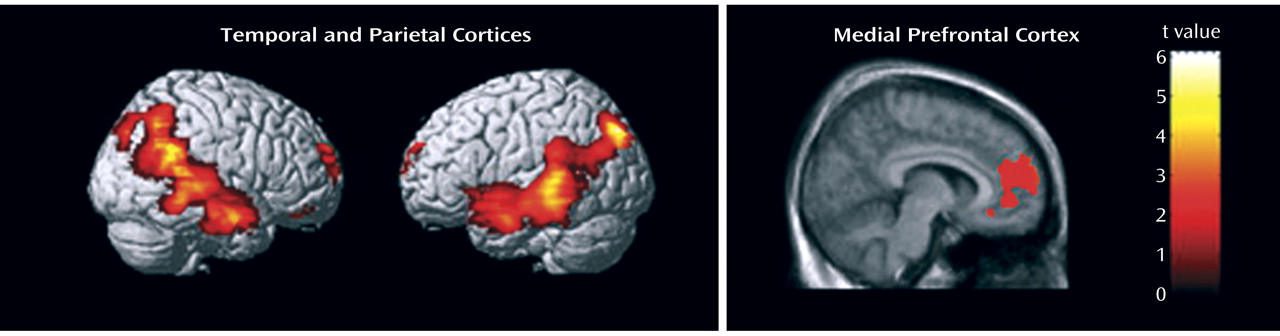
1 binding potential values compared with healthy comparison twins in the medial prefrontal cortex (+35%, F=8.74, df=1, 20.9, p=0.008), superior temporal gyrus (+38%, F=7.51, df=1, 18.2, p=0.013), and angular gyrus (+22%, F=5.90, df=1, 19.1, p=0.025). The independent voxel-based receptor parametric mapping analysis with correction for multiple testing confirmed these findings and showed significant clusters in the inferior parietal and lateral temporal lobes as well as the medial prefrontal cortex (
Figure 1 ). In unaffected co-twins, canonical liability scores were negatively correlated with D
1 binding potential in the putamen (N=9, R=–0.68, p=0.042), whereas spatial working memory performance was negatively associated with D
1 binding potential in the superior temporal gyrus (N=9, R=–0.48, p=0.045), suggesting that high D
1 binding potential was associated with poorer cognitive performance in these regions.
Patients with schizophrenia showed a significant widespread reduction in D
1 binding potential compared with their unaffected co-twins in almost all studied brain regions (see
Table 2 ; statistics available on request). No associations were observed between antipsychotic drug dose (in chlorpromazine equivalents) and D
1 binding potential in individual regions of interest, but a significant negative correlation was observed between drug dose and average D
1 binding potential of the frontal and temporal regions (partial R=–0.86, p=0.012, controlling for the effects of age and sex), indicating that high drug doses were associated with low D
1 binding potential in these regions.
A significant age effect was noted in the whole sample, with D 1 binding potential tending to decline with age in the angular gyrus (F=5.99, df=1, 13.1, p=0.029), caudate (F=7.49, df=1, 13.1, p=0.017), insular cortex (F=4.66, df=1, 14.7, p=0.048), putamen (F=5.20, df=1, 14.6, p=0.038), and supramarginal gyrus (F=6.79, df=1, 14.1, p=0.021). We observed a significant sex difference in the whole sample, with women having higher binding potential values than men in the medial prefrontal cortex (F=7.43, df=1, 12.2, p=0.018) and anterior cingulate cortex (F=5.75, df=1, 10.4, p=0.037). A significant hemisphere effect was observed in the inferior temporal gyrus (F=14.31, df=1, 40.3, p<0.001) and the middle temporal gyrus (F=7.28, df=1, 40.7, p=0.010). Left hemisphere values were higher than right hemisphere values in these regions. No significant group-by-hemisphere interactions were seen.
Moderate to high ICCs for binding potential in the healthy monozygotic twins were noted for both striatal and cortical regions, namely, the inferior temporal gyrus (0.84), putamen (0.80), middle temporal gyrus (0.80), caudate (0.71), supramarginal gyrus (0.66), and superior temporal gyrus (0.63). For other regions studied, ICCs were below 0.50.
Discussion
In this study, we used PET with [
11 C]SCH 23390 to investigate brain D
1 receptors in monozygotic and dizygotic twin pairs discordant for schizophrenia. The study produced two major findings. First, unaffected monozygotic co-twins of patients with schizophrenia showed increased D
1 receptor binding in the medial prefrontal cortex, superior temporal gyrus, and angular gyrus compared with healthy comparison twins. Monozygotic co-twins displayed the highest values compared with the comparison twins, while dizygotic co-twins had values intermediate between monozygotic co-twins and comparison twins, which suggests an association with increasing genetic loading for schizophrenia. To our knowledge, this is the first report of D
1 receptor alterations related to genetic risk for schizophrenia. The other major finding is that patients with schizophrenia who had experienced chronic antipsychotic treatment (probands in the discordant pairs) had a widespread reduction of D
1 receptor binding in the brain, which was associated with antipsychotic medication dose, a finding consistent with previous preclinical work
(18,
19) .
Binding potential represents a combined estimate of receptor density and apparent affinity, and the latter is affected by both the propensity of the radioligand to bind to the receptor and competition with endogenous dopamine for binding to the receptor. Acute fluctuations of endogenous dopamine do not change the in vivo binding of [
11 C]SCH 23390 in the striatum or cortex in humans
(26) or monkeys
(27,
28), and we interpret the results of our study as reflecting altered D
1 receptor density. It seems unlikely that the up-regulation of D
1 receptors in unaffected monozygotic co-twins observed in this study would be mediated by primary variations of the gene coding for the D
1 receptor. It is currently unknown whether some polymorphism in the D
1 receptor gene might affect receptor binding in vivo or even carry a risk for schizophrenia. SCH 23390 shows non-negligible in vitro affinity for serotonin 5-HT
2 receptors, and there is preliminary evidence from a recent nonhuman primate study
(29) that a small proportion of the cortical specific in vivo binding of both [
11 C]SCH 23390 (14%) and [
11 C]NNC 112 (28%) may originate from 5-HT
2A receptors. Human data are currently lacking for the in vivo pharmacological selectivity of these radioligands, but we cannot exclude the possibility that our findings may be due, to some extent, to hypothesized group differences in 5-HT
2A receptor density.
A novel finding of our study is the association of increased D
1 receptor density with increasing genetic risk for schizophrenia in the medial prefrontal cortex, superior temporal gyrus, and angular gyrus. This finding was confirmed with an independent voxel-based receptor mapping analysis. The interpretation of our finding of D
1 receptor binding elevation as an intermediate phenotype for schizophrenia is hindered by the fact that previous PET studies of D
1 receptor binding have produced contradictory findings, with studies showing decreased
(30), unaltered
(31), and increased
(32) binding in unmedicated patients compared with comparison subjects. These studies used different radioligands for D
1 receptors, namely [
11 C]SCH 23390
(30,
31) and [
11 C]NNC 112
(32) . The radioligand used may be important, since it has been shown that the cortical binding of [
3 H]SCH 23390 and [
11 C]NNC 112 behave differently following chronic dopamine depletion in rodents: [
11 C]NNC 112 binding increases, whereas [
3 H]SCH 23390 binding remains unchanged, a difference hypothesized to relate to differential affinity for internalized versus externalized receptors
(33) . Clearly, more studies are needed to validate these assumptions and to shed light on the discrepant findings in subjects with unmedicated schizophrenia.
Our second important finding was that patients with schizophrenia who had chronic antipsychotic treatment showed a profound and widespread reduction in D
1 receptor binding throughout the brain, including regions such as the striatum as well as frontal, temporal, and parietal cortices. Studies of nonhuman primates have shown that chronic administration of D
2 receptor antagonist medications down-regulates D
1 receptor binding in the frontal and temporal association areas but not in motor, visual, or somatosensory cortex or striatum
(18), as well as levels of D
1 and D
5 receptor mRNAs in the prefrontal cortex but not in the striatum
(19) . Consistent with this preclinical data, we found that high doses of antipsychotic medication were associated with low frontal and temporal D
1 receptor binding potential in the probands. Some of the antipsychotic drugs used by the patients in our study may also directly occupy D
1 receptors and thereby cause a reduction in [
11 C]SCH 23390 binding potential. Although the use of clozapine and flupenthixol was excluded, other drugs (such as thioridazine, which has only around a 10-fold D
2 /D
1 selectivity and was used by four patients) might contribute to a reduction in binding potential as well. However, we regard direct occupancy unlikely because a previous clinical PET study with medicated patients indicated that a daily dose of 300 mg of thioridazine did not induce detectable D
1 occupancy as assessed with [
11 C]SCH 23390
(21), and only one patient in our study had a daily dose over 300 mg. Because of this potential confounder, our results on our patient group are not directly comparable with previous D
1 receptor PET studies of unmedicated schizophrenia
(30 –
32) and are most likely reflective of D
1 receptor down-regulation, at least in the frontal and temporal cortices, induced by antipsychotic medication.
Dopaminergic hypofunction in the prefrontal cortex has long been regarded as one of the key neural substrates for deficit aspects of schizophrenia
(6), and a postmortem study has provided direct evidence for abnormal dopaminergic innervation of the medial prefrontal cortex in schizophrenia
(34) . The increased D
1 receptor density observed in our study might be viewed as a compensatory reaction to decreased cortical dopamine levels, which has been suggested to confer genetic liability for schizophrenia via polymorphisms in the gene coding for catechol
O -methyltransferase
(16) . Another hypothesis is that increased D
1 receptor binding is a compensatory phenomenon secondary to reduced neuropil
(35) —dendritic processes where D
1 receptors are preferentially located
(9) —which may be responsible for genetic vulnerability-related gray matter loss
(15) . The functional significance of D
1 receptors in the superior temporal gyrus and inferior parietal areas is currently unknown. Neurons in these regions express D
1 receptors
(36), and they participate in working memory functions in concert with neurons of prefrontal cortex
(37,
38), suggesting that they may be relevant for the pathophysiological process of schizophrenia. Consistent with the notion of marked inherited contributions, we observed high ICCs for striatal and parietotemporal D
1 receptor densities in the healthy monozygotic twins.
Sex differences in D
1 receptor density in the anterior cingulate cortex and medial prefrontal cortex are novel findings: women had higher receptor densities than men. Women have also been shown to have higher D
2 receptor binding in the anterior cingulate cortex
(39) . The significance of the sex difference in D
1 receptor density remains to be established in future studies. The observed significant lateralization in D
1 binding potential in the lateral temporal cortex is in line with the well-documented structural and functional asymmetry in these regions that has been attributed to the specialization of the left hemisphere to language functions
(40) .
One limitation of this study is the relatively small sample size. Nevertheless, the effect sizes for the group contrasts of interest were of sufficient magnitude to detect significant differences in cortical regions of theoretical importance in the pathophysiology of schizophrenia. It is important to emphasize, however, that with larger samples, similar effects may be detected in other brain regions.