Family, twin, and adoption studies have established that bipolar affective disorder has a genetic etiology, and the biological basis of the disorder has long been sought. Advancements have been made in identifying genes
(1) and biological markers
(2), but progress has been slow because of the heterogeneous nature of the clinical phenotype. The disorder varies greatly in severity, and clinical features that characterize one person’s illness may be uncommon or absent in another’s. This clinical heterogeneity may reflect heterogeneity at the genetic level, complicating efforts to map the involved genes.
Some of these clinical features are known to run in families. Previous work has shown that age at illness onset
(3), suicide
(4), psychosis
(5), and frequency of manic and depressive episodes
(6) cluster in families with bipolar affective disorder. While not everything that is familial is genetic, clinical features that do not run in families are unlikely to be informative for genetic studies
(7) . Clinical features that vary across individuals and run in families could reflect important genetic influences in those families. However, we have only an incomplete understanding of which clinical features are strongly familial in bipolar affective disorder and which are not.
One of the features of bipolar affective disorder that varies across individuals is the polarity of the initial episode at onset of illness. While symptoms of the disorder may be present from an early age, the first clear episode of bipolar affective disorder can be manic, depressive, or mixed in character. Individual variation in such an early feature of the illness may be a particularly promising phenomenon for genetic studies (8).
Polarity at illness onset is not only an early feature of bipolar affective disorder, but it also predicts the polarity of subsequent episodes. Turvey et al.
(9), in a 15-year follow-up study of 165 probands with bipolar affective disorder, found that polarity at onset predicts the polarity of future episodes, even when subsequent episodes switch polarity one or more times before remission. Among individuals whose illness started with mania, 75% of subsequent episodes studied started with mania, and among individuals whose illness started with a depressive episode, 55%–60% of subsequent episodes started with depression. Thus, the initial polarity may have implications for the lifetime course of bipolar affective disorder.
Polarity at onset has also been shown to be correlated with severity of illness and with treatment response. Kukopulos et al.
(10) reported that individuals whose illness follows a mania-depression-free interval sequence have a better response to lithium than those with a depression-mania-free interval sequence. Similarly, Haag et al.
(11) concluded that subjects with a mania-depression-free interval sequence are more likely to benefit from lithium treatment, possibly reflecting a “difference in the underlying mechanisms of dysregulation.” Similar conclusions were reached by Maj et al.
(12), and a review of five studies of onset polarity and polarity sequence noted the consistency of findings across studies
(13) . Recently, Perlis et al.
(14) reported that individuals who had major depression at onset reported an earlier age at onset and more comorbid anxiety disorders, and they were more likely to attempt suicide. Taken together, these studies suggest that polarity at onset may reflect differing biological pathways leading to differing severity and treatment response in bipolar affective disorder.
We assessed polarity at onset among 971 individuals with bipolar affective disorder in 507 families ascertained and diagnosed by the NIMH Genetics Initiative Bipolar Disorder Consortium. In this study we addressed four main questions: 1) Does polarity at onset run in families? 2) How similar are relatives in their polarity at onset? 3) Does polarity at onset correlate with other familial traits in bipolar affective disorder? 4) Does polarity at onset define subtypes of bipolar affective disorder that are genetically informative?
Method
Subjects
As part of a large multisite genetic linkage study, detailed clinical data were collected on 600 families ascertained through sibling pairs affected with bipolar I disorder or schizoaffective bipolar disorder. Ninety-three of these families were excluded from this analysis because of missing data on age at onset of depressive and manic episodes. We studied the remaining 507 families, which comprised a total of 971 affected individuals.
Clinical Assessment and Diagnosis
Subjects were interviewed with the Diagnostic Interview for Genetic Studies, versions 1–3
(15), administered by trained interviewers. This instrument collects information on ages at onset, clinical features of the most severe episodes of major depression and mania, lifetime suicidal behavior, lifetime comorbid conditions such as alcohol abuse and panic disorder, and a variety of other data. Hospital notes were also obtained when possible, and the Family Interview for Genetic Studies
(16) was used to collect collateral information from at least one other family member. Data were reviewed by two clinicians who independently assigned diagnoses in a best-estimate procedure. Any diagnostic disagreements were resolved by a third reviewer.
Polarity at Onset
Polarity at onset was determined by comparing the reported ages at onset for the first major depression and the first mania. Subjects were categorized into three groups: 1) those designated as having major depression at onset because their age at onset of major depression was lower than that of mania; 2) those designated as having mania at onset because their age at onset of major depression was greater than that of mania; and 3) those designated as having major depression and mania at onset because they experienced episodes of both during the initial year of illness. The major depression and mania group may include individuals with mixed episodes, rapid cycling, biphasic episodes, and frequent episodes with recovery in between, but our data do not allow us to distinguish among these features.
Validity of age at onset was assessed by comparing the earliest age at onset reported in the Diagnostic Interview for Genetic Studies to that assigned by the best-estimate clinicians. The two values were highly correlated (r=0.973, p<0.001), and the age at onset reported in the interview was within 1 year of the best-estimate value in almost all cases, which suggests that using the ages at onset recorded in the interview appears to be a reasonable way to establish polarity at onset in this data set.
Statistical Analysis
A mixed-effects regression model for nominal variables (MIXNO) was applied to test familial clustering of polarity at onset
(17) . Log likelihoods were compared between a model with family membership as a random factor and an intercept-only model that considered all subjects together without regard to family membership. Gender was used as a covariate in both models.
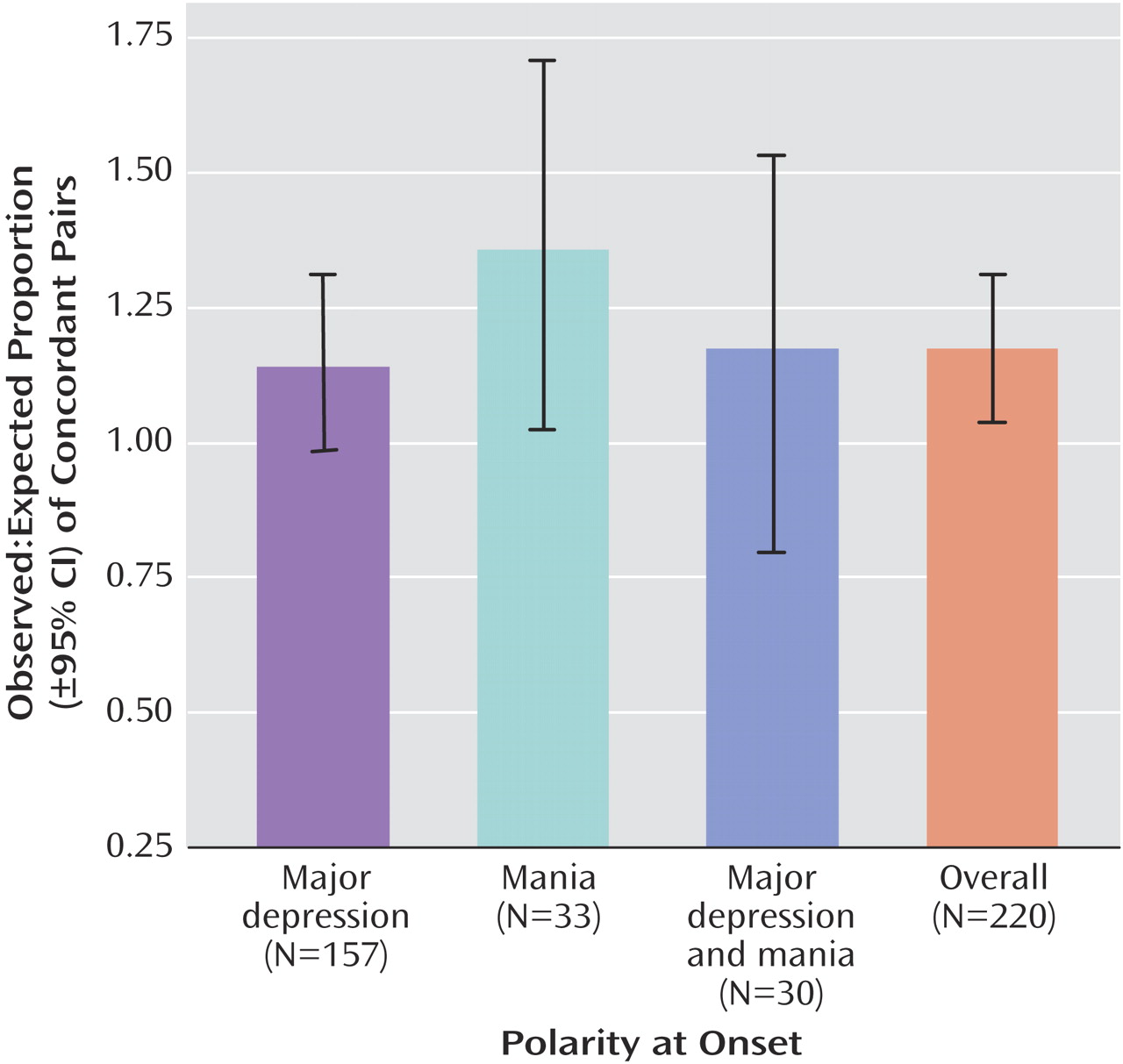
To further assess the strength of familial clustering, we measured concordance of polarity at onset among independent affected relative pairs. Each of n affected individuals in a family was paired with one random affected relative to generate n–1 affected relative pairs per family. Concordance was calculated by dividing the number of pairs concordant for mania at onset, major depression at onset, or both by the total number of pairs. Expected concordance rates for each onset polarity were estimated as the square of the frequency of that polarity at onset in the total sample
(18) .
We assessed the relationship between polarity at onset and several other commonly studied clinical features: age at onset, number of major affective episodes, psychosis, alcoholism, panic attacks, and suicide attempts. We defined age at onset as the first episode of mania or of major depression, whichever came first
(19) . Major affective episodes were counted as the number of major depressive or manic episodes not attributable to a medical condition, bereavement, or substance use. Psychosis was defined as lifetime history of hallucinations or delusions, although in this sample psychotic symptoms were usually confined to manic or depressive episodes. Panic attacks were defined as lifetime history of attacks meeting DSM-III-R criteria for panic. Alcoholism was defined as alcohol use meeting DSM-III-R criteria for abuse or dependence. Suicide attempt was defined as a lifetime history of self-harm with intent to die.
Analysis of variance was used to assess the relationship between polarity at onset and continuous variables; specific comparisons were performed with the Tukey honestly significant difference test. Age at onset, episode frequency, and number of depressive episodes were log-transformed to ameliorate their highly skewed distributions. The relationship between polarity at onset and binary variables was assessed with the chi-square test. All p values were two-tailed.
Linkage analysis was conducted using the lodpal program in the Statistical Analysis of Genetic Epidemiology (SAGE) package, version 5.1 (Case Western Reserve University, 2005). Polarity at onset was used as a covariate. Each pair of relatives was assigned a score reflecting the polarities at onset of the individuals in the pair. To preserve sample size, individuals whose illness started with both mania and major depression were scored concordant with any polarity at onset. Linkage was assessed under models that did and did not incorporate the covariate. Empirical significance was determined by permuting the covariate values 1,000 times and tallying the scores that exceeded those seen in the real data. For proof of concept, we tested only two chromosomes, 6 and 16, both of which contain loci that have been linked to bipolar affective disorder in the NIMH Genetics Initiative sample
(20,
21) . Genetic marker data and marker maps were generated by the Center for Inherited Disease Research and screened for errors
(22) .
Results
General
Of the 971 affected individuals in our study, 65% (N=631) were female. A total of 524 subjects (54%) reported a major depressive episode at illness onset, and 223 (23%) reported a manic episode; for these subjects, the median time between age at onset episode and age at first episode of the opposite polarity was 4 years. A total of 223 (23%) subjects reported having both major depression and mania during the initial year of illness.
Familiality
Polarity at onset was significantly familial in this sample. The model that incorporated family membership as a random effect was significantly more predictive than the alternative model (log-likelihoods, –975.520 and –978.372, respectively; log-likelihood ratio=1.002 [a value >1 favors the model with family as a random effect]; likelihood ratio statistic=5.704, df=1, p<0.017). Relatives were about twice as likely to have the same polarity at onset as they were to experience a different polarity at onset. In the pairwise analyses, observed concordance for polarity at onset was greater than the expected concordance. Breakdown by polarity at onset indicated a particular excess of relative pairs concordant for mania at onset (
Figure 1 ).
Associated Clinical Features
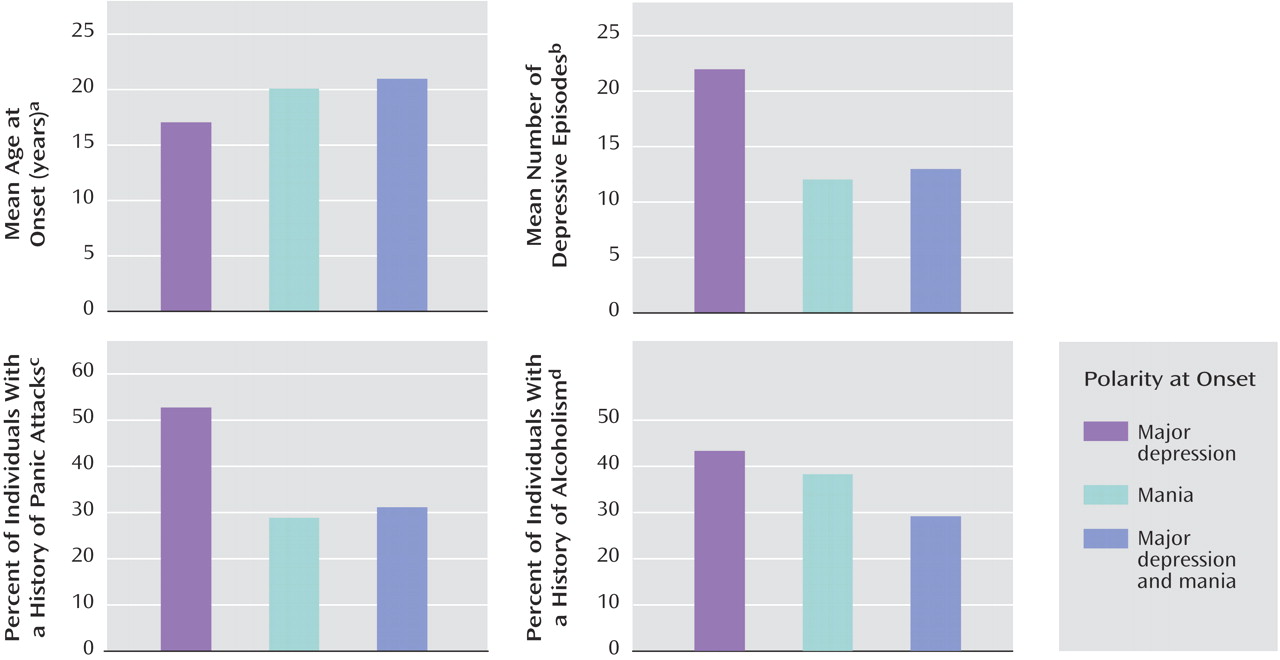
There was a significant relationship between age at onset and polarity at onset (
Figure 2 ). Specific comparisons indicated that age at onset for individuals whose illness started with mania was 3 years higher on average than for those whose illness started with major depression.
We also examined the lifetime number of manic and depressive episodes. There was a significant relationship between polarity at onset and number of major depressive episodes (
Figure 2 ). Subjects whose illness started with mania reported fewer depressive episodes than subjects whose illness started with major depression. Subjects who reported major depression at onset had approximately twice as many major depressive episodes as subjects who reported mania or both mania and depression at onset. In contrast, there was no significant relationship between polarity at onset and lifetime number of manic episodes.
Polarity at onset was associated with some of the categorical variables we tested, but not all. There was a significant association between polarity at onset and panic attacks (
Figure 2 ). Compared with subjects who had mania at onset or had both major depression and mania during the onset year, 58% more subjects who had major depression at onset reported a history of panic attacks.
Alcoholism and polarity at onset were also significantly associated (
Figure 2 ). Compared with subjects who had mania at onset or who had both major depression and mania at onset, 45% more subjects who had major depression at onset met DSM-III-R criteria for alcohol abuse or dependence.
The remaining variables we tested showed no significant association with polarity at onset in this sample. There was a somewhat greater prevalence of suicide attempts among those who had major depression at onset, but this association did not reach statistical significance. There was no association between gender and polarity at onset. There was no association between psychosis and polarity at onset. About one-third of subjects in each onset polarity category reported a history of psychosis.
Linkage to Chromosomes 6 and 16
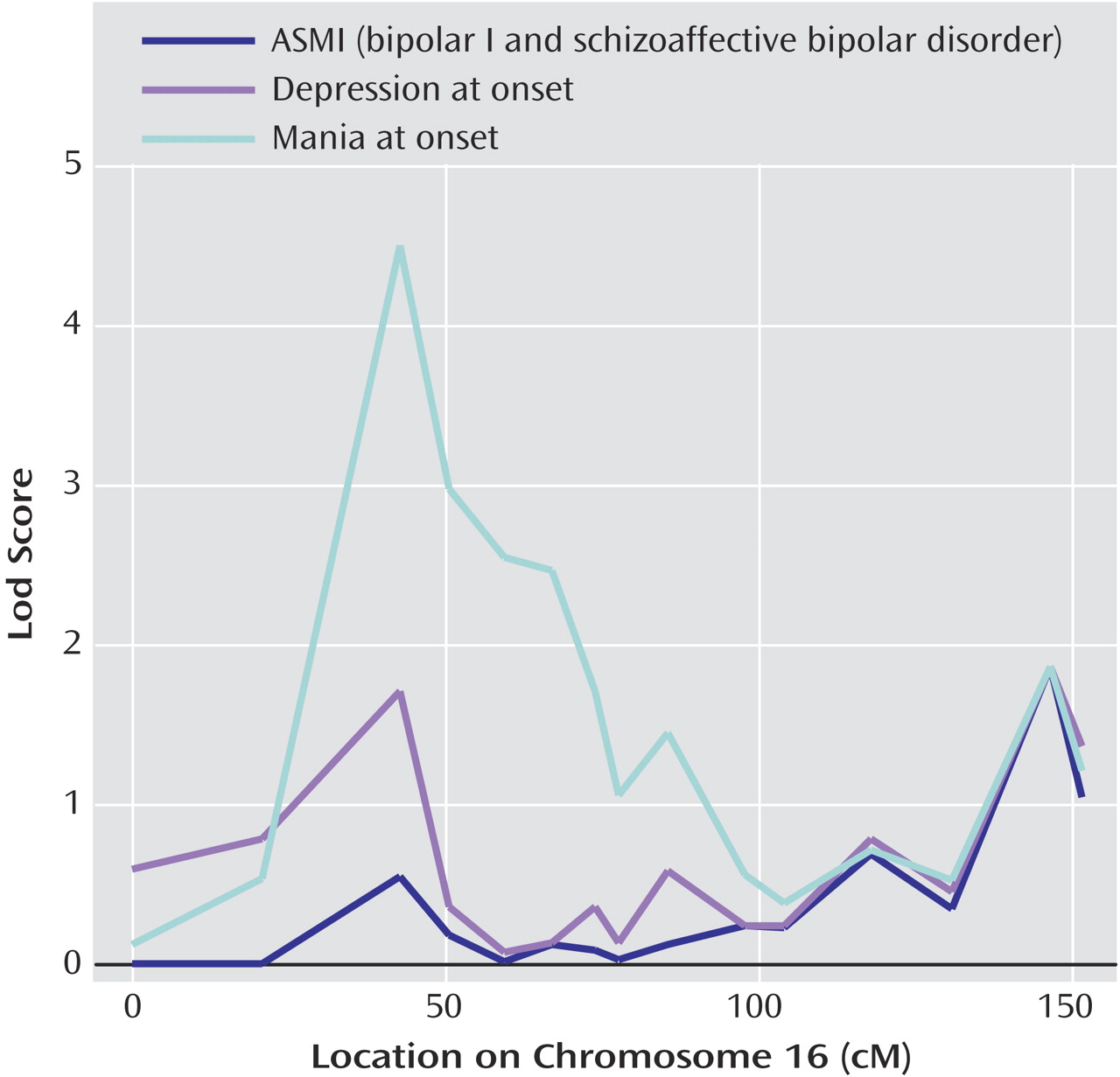
Linkage analysis, incorporating polarity at onset as a covariate, revealed significant evidence of linkage to chromosome 16p (
Figure 3 ). The conditional lodscore peaked at 4.5 at D16S748 under the covariate scoring that weighted relative pairs concordant for mania at onset (empirical p=0.004). No significant increase in evidence of linkage to chromosome 16 was detected under the scoring that weighted relative pairs concordant for major depression at onset. Polarity at onset was not associated with linkage to chromosome 6 in this sample (data not shown).
Discussion
To our knowledge, this is the first demonstration that polarity at onset is a familial trait in bipolar affective disorder. Previous studies have shown that polarity at onset is predictive of clinical course and treatment response. Our results confirm and extend these findings, demonstrating that polarity at onset runs in families and is related to age at onset, number of depressive episodes, alcoholism, and panic attacks. Our results also suggest that polarity at onset, particularly mania at onset, may be genetically informative. Thus, polarity at onset delineates forms of bipolar affective disorder with distinct clinical features that may be useful for genetic and other biological studies.
Our data are subject to several limitations. Determination of polarity at onset in our sample was based on retrospective self-report of the ages at first major depression and first mania. Such data are known to be subject to recall bias, even when elicited by trained interviewers
(23,
24) . However, relatives are not correlated for recall bias
(25) . Furthermore, there was a median of 4 years between age at onset episode and age at first episode of the opposite polarity in this sample. Thus any imprecision in reported ages at onset would need to be substantial in order to change the polarity-at-onset classification; such imprecision would only tend to diminish apparent familiality and any differences between groups classified by polarity at onset. In contrast, we found that polarity at onset was strongly familial and predicted significant differences in several important clinical features. Thus, it does not appear that recall bias could account for our findings.
Our sample was largely opportunistically ascertained on the basis of sibling pairs affected with bipolar I or schizoaffective disorder and hence cannot be considered representative of a community sample. While this ascertainment might be expected to increase the proportion of subjects who have mania at onset, the majority of our subjects actually reported major depression at onset of their illness. Thus, it does not appear that our ascertainment method can account for our findings.
We observed significant similarity in polarity at onset between relatives, which was largely attributable to a greater-than-chance concordance among relatives for mania at onset. The demonstration that polarity at onset runs in families does not prove that it has a genetic basis. It is possible that aspects of the shared family environment contribute to the resemblances we observed among relatives. A twin study design in which concordance in dizygotic twins is compared with that in monozygotic twins would be one way to disentangle genetic and shared environmental influences on a trait. To our knowledge, no twin studies have examined polarity at onset in bipolar affective disorder. However, in the course of collecting the NIMH Genetics Initiative sample, 27 pairs of monozygotic twins and 31 pairs of dizygotic twins were studied. Although this is a small and unrepresentative sample by twin-study standards, concordance in polarity at onset does appear to be greater in the monozygotic than in the dizygotic twins (data not shown), which suggests that at least some of the familial resemblance we observed in polarity at onset has a genetic basis.
As a test of the hypothesis that greater clinical homogeneity corresponds to greater genetic homogeneity, we reanalyzed the genetic linkages to chromosomes 16p and 6q previously detected in this sample
(20 –
22), this time using polarity at onset as a covariate in the linkage test. Linkage analysis with covariates can provide greater power to detect linkage when the covariate is a marker of locus heterogeneity in the sample. Our results indicated increased evidence of linkage to 16p when mania at onset was used as a covariate. This was consistent with our finding that the familiality of polarity at onset was driven mainly by a greater prevalence of relative pairs concordant for mania at onset in this sample. No increased evidence of linkage was detected on chromosome 6. This finding suggests that mania at onset does not delineate some generally more genetic form of bipolar affective disorder. Rather, it appears that polarity at onset is simply not a marker of heterogeneity at the 6q locus.
Our results demonstrated additional clinical differences between individuals who had major depression at onset and those who had mania at onset. Subjects with mania at onset had a later age at onset and fewer major depressive episodes over the course of their illness. They were also less likely to suffer from panic attacks or alcoholism. These findings are consistent with previous studies demonstrating a more benign course in bipolar affective disorder with mania at onset
(9 –
15) . Some of these studies attribute the more benign course to a better response to lithium, but we cannot test that hypothesis with this sample because we have no data on lithium response. Other studies have suggested that a higher age at onset tends to be associated with a less severe form of bipolar affective disorder
(3,
24), which is consistent with our findings.
Subjects who had both major depression and mania during their initial year of illness seem to occupy an intermediate position in this sample, at least with regard to panic attacks and alcoholism. As noted, however, these subjects probably constitute a mixed group, encompassing individuals with a variety of clinical profiles that cannot be distinguished in our data. In additional analyses, we found that these subjects indeed have a higher frequency of manic and depressive episodes over the course of their illness than do those who have mania at onset (F=3.846, df=2,879, p=0.022; data not shown). Only a minority of relative pairs were concordant for this type of onset, however.
Polarity at onset may delineate forms of bipolar affective disorder that have distinct clinical courses. We have shown that polarity at onset is a familial component of bipolar affective disorder associated with important clinical features. We have also demonstrated that polarity at onset may have genetic validity, since in linkage analysis mania at onset increased evidence of linkage to chromosome 16p in this sample. Thus, polarity at onset may help define subtypes of bipolar affective disorder that are more homogeneous in clinical features and in underlying genetic etiology.