Findings over the last decade have demonstrated that morning bright light exposure ameliorates symptoms of seasonal affective disorder when gauged against nonphotic placebos
(1,
2) . Concurrently, basic biological rhythm research has pointed to dimmer gradual naturalistic dawn and dusk simulation as a potent alternative to bright light exposure. For example, hamsters show stronger circadian entrainment to non-24-hour light-dark cycles under naturalistic twilights than under conventional rectangular transitions
(3) . Rats self-select the dimmer twilight signal to maintain circadian entrainment when given the opportunity to escape daylight exposure
(4) . The rat retina responds to naturalistic dawn simulation with accelerated shedding of rod outer disk segments compared with sudden light onset
(5) . In the human laboratory, naturalistic dawn simulation prevents the delay drift of rhythms under dim light conditions
(6) . Bedside administration of a naturalistic dusk-to-dawn signal advances the sleep episode in demented elderly, with a tendency toward reduced sleep latency, longer duration, and decreased nocturnal activity
(7) .
In identifying early morning bright light exposure as more effective than later morning or evening light for patients with seasonal affective disorder
(8), our attention was drawn to the early dawn interval, when melatonin wanes, core body temperature begins to rise, and the circadian timing system has the greatest propensity for light-elicited phase advances
(9) . In the wintertime at northerly latitudes, this is also the period when it remains dark outdoors, a putative trigger of the depression. Therefore, we set out to explore the antidepressant effects of simulated dawn illumination during the final hours of sleep
(10) .
Although we previously demonstrated effective treatment for winter depression by using naturalistic dawn simulation in a case series
(11,
12), until now it has not been tested against control subjects given placebo or compared directly with postawakening bright light exposure. However, Avery and associates
(13 –
15) have investigated a variant of dawn simulation in hypersomnic patients with seasonal affective disorder with a 90-minute sigmoidal illumination ramp with accelerated brief exposures and dim red exposures as controls. They found the sigmoidal simulation superior to postawakening bright light therapy
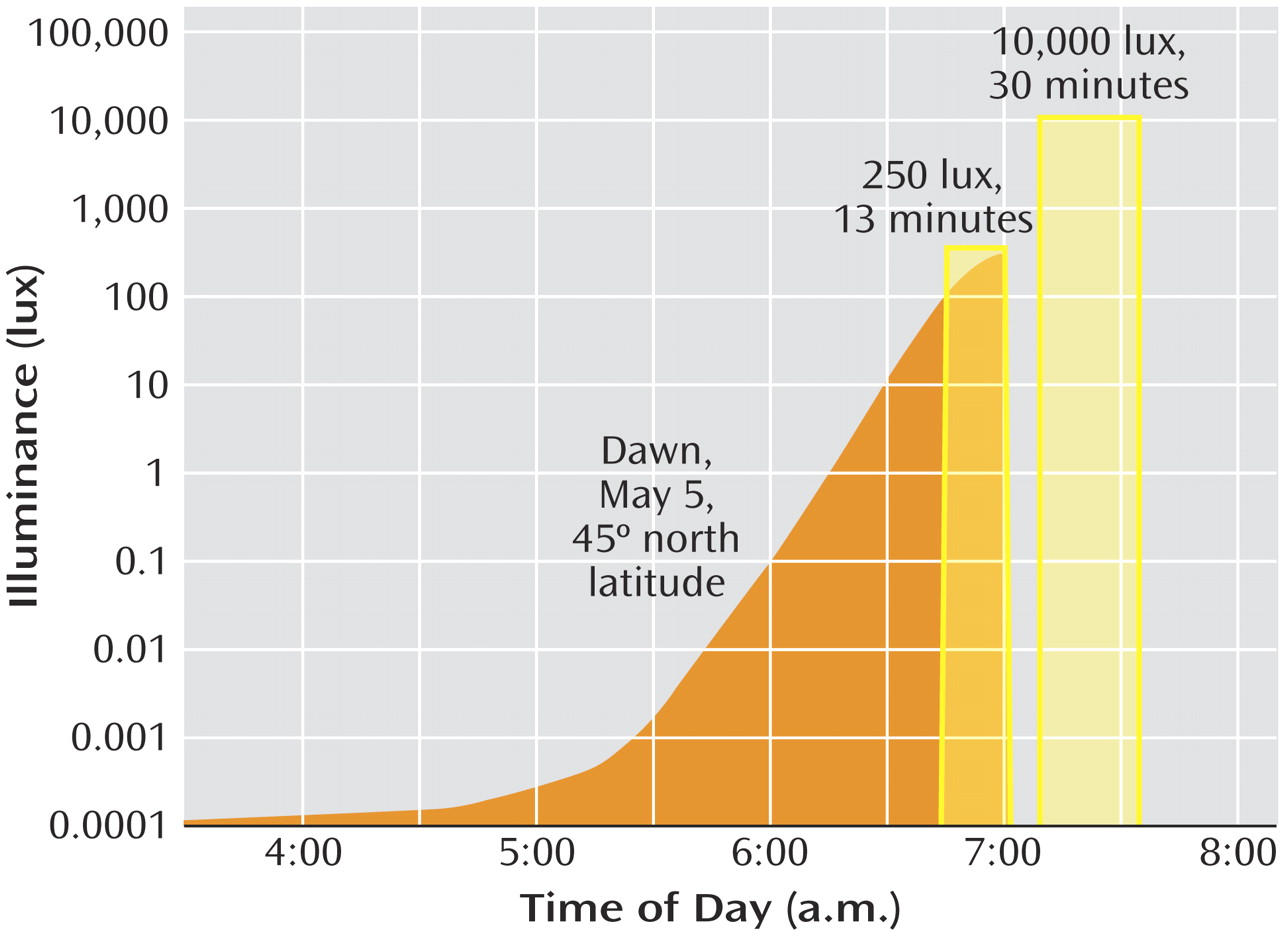
(15) . However, some patients experienced side effects that we had not observed with naturalistic dawns: premature awakening during the initial exposure to the rising signal, occasionally accompanied by hypomania. The sigmoidal signal contrasts with naturalistic dawns, which begin several hours earlier in astronomical twilight and rise more gradually (
Figure 1 ).
In the present study, we examined the efficacy of high-density ion exposure and naturalistic dawn simulation, both presented toward the end of sleep. We compared both methods with low-density negative air ionization (as a placebo) and postawakening bright light (as an established effective treatment). Additionally, as a control for the gradual naturalistic dawn signal, we presented a brief sunrise pulse, matched in total illuminant dose, just before wake-up time.
Results
During the 6 years of the study (conducted November to March), 126 subjects entered the study and 118 (93.7%) completed it. The noncompleters all withdrew before the 10-day midpoint evaluation, two for noncompliance (low-density ions, one; bright light, one), with six dropouts (low-density ions, one; high-density ions, three; bright light, one; and dawn simulation, one). Of the completers, 19 who experienced remission (16.1%) did not show relapse during the withdrawal phase; they were distributed across all five groups with no significant differences (Fisher’s exact test). Because such sustained improvement cannot be distinguished from spontaneous seasonal remission, they were excluded according to protocol
(2,
24) from the final data set. The results were analyzed for 99 subjects who either remained depressed at treatment endpoint or showed relapse during the withdrawal phase. The group included 77 (77.8%) women and 22 (22.2%) men, ages 19–63 years (mean=40.4 years, SD=10.4). The distributions of age and gender were closely balanced, with no significant differences by univariate analysis of variance and Fisher’s exact test, respectively. The diagnoses were major depressive disorder in 94 (94.9%) of the cases and bipolar disorder not otherwise specified or bipolar II disorder in five (5.1%) of the cases.
Rating Scale Means and Percentage Improvement
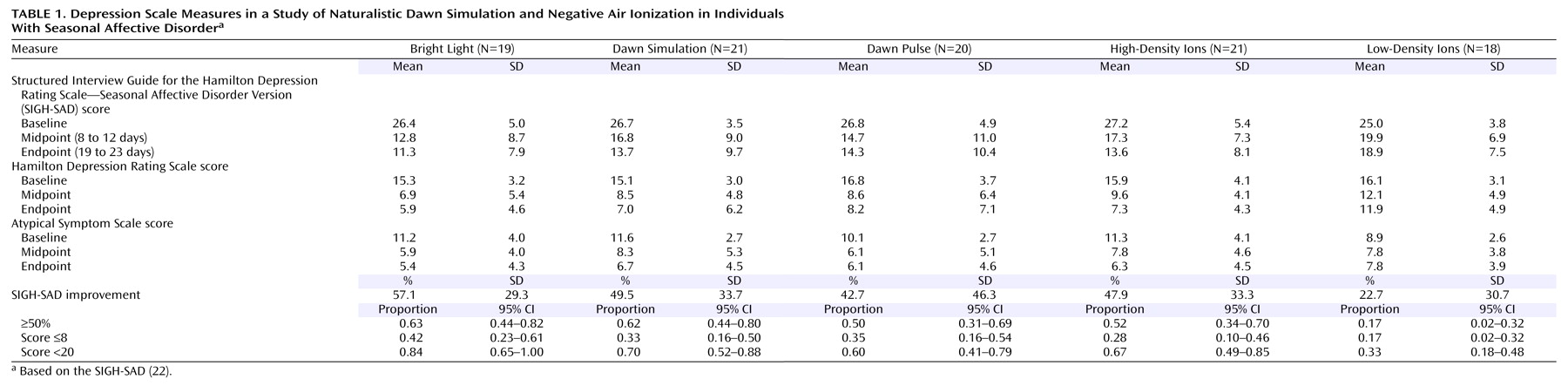
The overall mean SIGH-SAD score at baseline was 26.5 (SD=8.0), with an HAM-D mean score of 15.8 (SD=4.9) and an Atypical Symptom Scale mean score of 10.6 (SD=3.3). Univariate ANOVAs showed no significant baseline differences among the five groups (
Table 1 ). A repeated measures ANCOVA of SIGH-SAD scores across the three assessment points, with baseline score, age, and gender as covariates, revealed the following significant effects: group (F=2.48, df=4, 91, p=0.05), time (F=3.60, df=2, 90, p=0.03), and group-by-time interaction (F=2.51, df=4, 91, p=0.05). There were no significant effects of age (F=0.38, df=1, 91, p=0.94) or gender (F=1.02, df=1, 91, p=0.32). Improvement was greatest between baseline and the 10-day midpoint, with little change between midpoint and endpoint (
Table 1 ). Least significant difference comparisons between groups showed that the percentage improvement for the low-density ion group was significantly lower than for the four alternate groups (p=0.001 to p=0.02), and there were no significant differences among the latter groups. Posttreatment improvement was far lower for the low-density ion group (22.7%) than for the alternate groups (42.7% to 57.1%). The ANCOVA for raw scores also revealed significant effects of the baseline score covariate (F=14.29, df=1, 91, p<0.001) and the baseline score-by-time interaction (F=13.98, df=2, 90, p<0.001), which reflected greater opportunity for score reduction in more severe cases (r=0.28, N=99, p=0.004)
(25) .
ANCOVAs on the scores from the HAM-D and Atypical Symptom Scale yielded contrasting results. The HAM-D showed the following significant effects, mirroring results for the SIGH-SAD: group (F=2.97, df=4, 91, p<0.03), time (F=4.19, df=2, 90, p<0.02), group-by-time interaction (F=2.99, df=4, 91, p<0.03), baseline score covariate (F=14.23, df=1, 91, p<0.001), and baseline score-by-time interaction (F=21.09, df=2, 90, p<0.001). On the Atypical Symptom Scale, however, the only significant effects were for the baseline score covariate (F=65.15, df=1, 91, p<0.001) and the baseline score-by-time interaction (F=15.23, df=2, 90, p<0.001). Nonsignificant effects included group (F=1.96, df=4, 91, p=0.11), time (F=1.36, df=2, 90, p=0.26), and the group-by-time interaction (F=2.02, df=4, 91, p=0.10). When we used percentage improvement rather than raw scores, the Atypical Symptom Scale did show a significant group effect (F=2.76, df=4, 91, p=0.03) that isolated low-density ions as less effective treatment, also seen on the HAM-D (F=3.16, df=4, 91, p=0.02). The two scales showed similar magnitudes of improvement except under low-density ions (HAM-D: mean=29.2%, SD=39.7%; Atypical Symptom Scale: mean=3.9%, SD=56.3%) (t=1.95, df=34, p=0.06, two-tailed), which may indicate a blunted placebo response for the symptoms of hypersomnia, hyperphagia, and fatigue. Further analyses focused on the combined SIGH-SAD scale.
Categorical Measures of Treatment Response
The proportion of patients achieving 50% or greater improvement (
Table 1 ) differed significantly between groups (χ
2 =10.55, df=4, p=0.03) and was far lower under low-density ions than under the other conditions (which did not differ significantly from each other). The proportion of patients meeting the remission criterion of a SIGH-SAD score of 8 or lower followed a similar pattern, although the groups did not differ significantly (χ
2 =3.15, df=4, p=0.53), which may reflect the higher remission rate for low-density ions (mean=0.17, SD=0.15) than in our previous parallel group study (mean=0.05, SD=0.09)
(2) . The proportion with posttreatment scores below the 20-point baseline entry level, however, varied significantly (χ
2 =11.37, df=4, p=0.02), with the low-density ion group falling lower than the others.
Residual Symptoms, Emergence, and Exacerbation
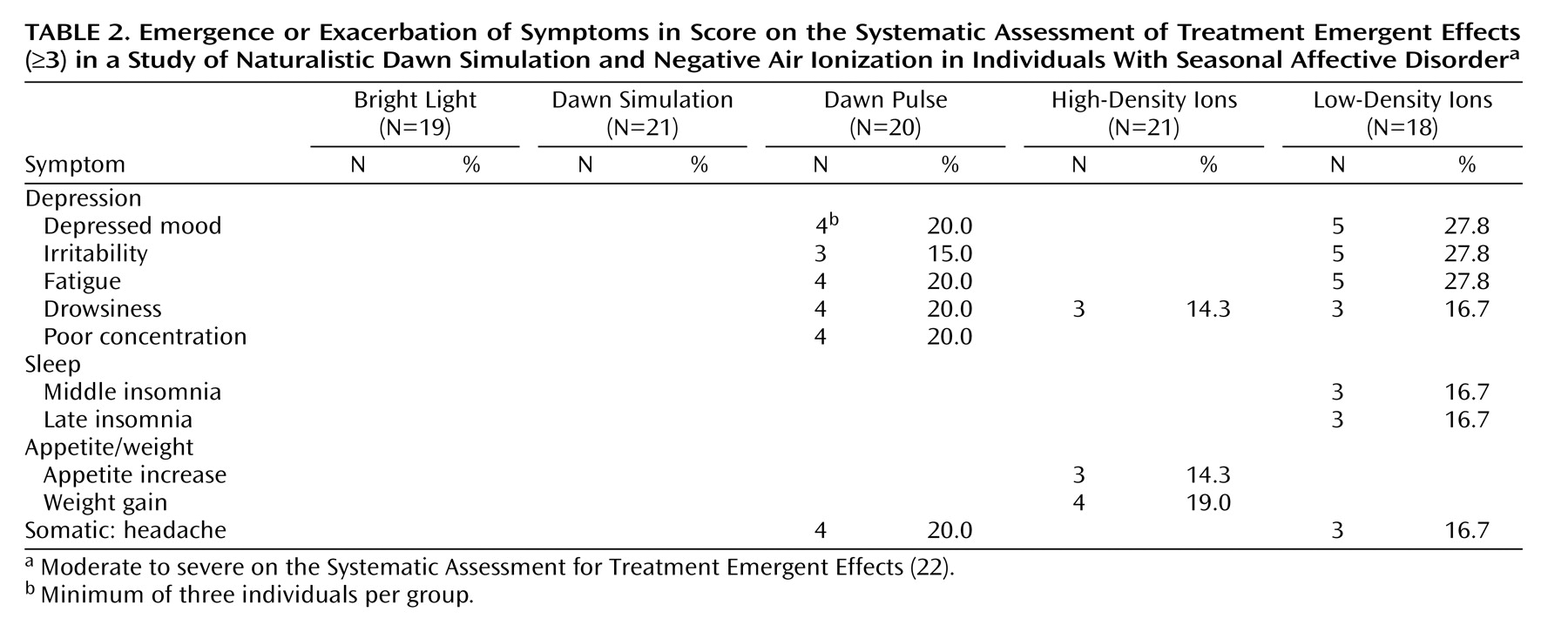
Of 88 potential somatic and psychological side effects tabulated by the Systematic Assessment for Treatment Emergent Effects, the only ones to show posttreatment emergence or exacerbation to moderate or high severity fell into the depression cluster (
Table 2 ). The patients reporting these symptoms were all nonresponders who showed the same symptoms at baseline; thus, none of these symptoms was emergent, and all reflected exacerbation under ineffective treatment. Accordingly, they occurred most often under low-density ions. The dawn pulse produced a profile very similar to that for low-density ions, although poor concentration was reported only by patients under the dawn pulse, and sleep disturbance was reported only under low-density ions. When residual and exacerbated symptoms were combined, a significantly higher proportion of the patients reported disturbance under the dawn pulse than under dawn simulation (mean=0.50, SD=0.19, versus mean=0.19, SD=0.14) (χ
2 =4.36, df=1, p=0.04).
Because the Systematic Assessment for Treatment Emergent Effects does not assess suicidality, we analyzed baseline and endpoint ratings for the HAM-D item. There were only two cases of emergence or exacerbation, both under the dawn pulse. One patient who scored 0 points at baseline scored 2 points (“wishes he were dead or any possible thoughts of death to self”) at the end of treatment, whereas the second patient moved from a score of 2 to 3 points (“suicidal ideas or gesture”).
Expectations
Mean expectation ratings differed across groups by less than 1 point on the 5-point scale: bright light, 3.5 (SD=0.9); dawn signals, 3.3 (SD=0.7); and ions, 2.7 (SD=0.9) (F=8.27, df=2, 96, p<0.001). Comparisons of the least significant difference showed that both the bright light and the dawn groups had higher expectations than the ion group. Of importance, expectations were not significantly different between the dawn simulation and dawn pulse subgroups (mean=3.4, SD=0.7, versus mean=3.1, SD=0.8) or the low- and high-density ion subgroups (mean=2.8, SD=1.0, versus mean=2.5, SD=0.9). Overall, expectation ratings were significantly correlated with endpoint SIGH-SAD percentage improvement (r=0.36, N=99, p<0.001), which was also observed separately in the three light groups (r=0.28, N=60, p=0.03) and the two ion groups (r=0.37, N=39, p=0.02).
Discussion
The trial design provided for several tests of efficacy, following the hypotheses that both dawn simulation and high-density ions would produce greater antidepressant response than low-density ions and that dawn simulation would be superior to both the dawn pulse and bright light treatment. Analysis of raw depression scale scores showed that dawn simulation and high-density ions were superior to low-density ions. However, the responses to bright light therapy and dawn pulse did not differ significantly from the response to dawn simulation. Percentage improvement and categorical measures of response and remission showed similar patterns. On this basis, we concluded that 1) naturalistic dawn simulation provided no advantage (other than convenience of use) over postawakening bright light therapy and 2) the dawn pulse is an effective treatment when we consider its superiority to low-density ions. Thus, it appears that gradual twilight is not necessary for therapeutic action during sleep. There is a partial precedent for such a dawn pulse effect in an uncontrolled trial of remitted depressed patients with residual hypersomnia
(26) . When the patients were briefly exposed to 500 lux incandescent light switched on by a timer 10 minutes before the desired wake-up time, they reported easier awakening and a shortened sleep duration.
Despite the superiority of the dawn pulse over low-density ions and lack of difference from the other active treatments, overall response to the pulse was undermined by a distinct group of nonresponders. The Systematic Assessment for Treatment Emergent Effects ratings showed a pattern of exacerbation of depressive symptoms under the dawn pulse similar to that under low-density ions. Furthermore, HAM-D ratings showed two cases of emergent or exacerbated suicidality (both without active intent). By contrast, dawn simulation showed no such problems. We conclude that although the dawn pulse is therapeutically active in some patients, the risk of symptom persistence and emergence and exacerbation in other patients makes it an unfavorable option.
Several potential dosing parameters of naturalistic dawn simulation—dawn pulse, bright light, and negative air ions—might change their relative efficacy. For dawn simulation, there are the choices of day of year (solstices slowest, equinoxes fastest), illuminance anchor at sunrise (unshielded sunrise provides approximately 800 lux), sunrise time anchor relative to habitual wake-up time, spectral composition of the signal, and duration of the signal after sunrise. An additional factor for the dawn pulse is its duration preceding wake-up. Apart from spectral characteristics
(27), the duration and intensity of bright light therapy
(24) and its timing relative to the individual’s circadian rhythm phase
(28) are known to affect remission rate. As for negative air ions, the effects of flow rate (resulting in proximal ion density) and timing and duration of exposure have yet to be explored.
Expectation ratings for ions in our study were slightly but significantly lower than the ratings for light, which raises the question of the adequacy of low-density ions as a placebo control. Several results mitigate this potential confound. First, the low-density ion group showed no significant correlation of expectation ratings with SIGH-SAD percentage improvement (r=0.16, N=18, p=0.53). Second, the ratings did not differ significantly between the low- and high-density ion groups, yet the response was far greater to the high-density ions. Third, the response to high-density ions did not differ significantly from that for the light groups.
As in our previous trials of light therapy
(24) and negative air ionization
(2), we used a strict criterion for data entry into the primary analysis, observation of relapse to the minimum baseline SIGH-SAD score of 20 within 3 weeks of discontinuation for the patients who showed remission during the treatment phase. Two factors support the exclusion of nonrelapsers. First, maintained improvement after treatment discontinuation during the expected period of a major depressive episode is prima facie evidence of a placebo response
(29) . Especially in studies with a relatively small group size, higher placebo rates can seriously reduce the power to detect significant differences. This is less of a problem in larger drug trials with hundreds of patients with seasonal affective disorder
(30), which have been economically infeasible for nondrug alternatives
(31) . Second, within a time-limited winter episode, it is impossible to know whether maintained remission after discontinuation reflects spontaneous seasonal improvement or a response to active treatment. We have reported universal relapse after treatment early in the winter season
(32) . It is possible that prior light therapy trials that failed to find superiority over placebos would have concluded differently if relapse during a withdrawal phase had been ascertained and nonrelapsers excluded. To test this supposition, we conducted a post hoc analysis of SIGH-SAD improvement of 50% or greater, including all patients, regardless of relapse. Perforce, response rates increased, especially for low-density ions. Although the pattern of group contrasts was retained, it fell short of statistical significance: bright light, mean=0.67, SD=0.17; dawn simulation, mean=0.67, SD=0.17; dawn pulse, mean=0.63, SD=0.17; high-density ions, mean=0.57, SD=0.17; low-density ions, mean=0.35, SD=0.17) (χ
2 =6.75, df=4, p=0.15). Similarly, ANCOVAs on raw scores fell short of statistical significance.
The hypothesis that dawn simulation is superior to bright light therapy has been attractive because of the springtime pattern of illumination, which is lacking in winter
(10) . However, the hypothesis becomes less attractive given the similarity of response to dawn and postawakening bright light in our study. Like Avery and associates
(13 –
15), we showed that a dawn signal presented toward the end of sleep was superior to that of placebo in comparison subjects (in their case, dim or brief light ramps with lower illuminant dose; in our case, low-density negative air ionization), also presented during sleep. Unlike Avery and associates
(15), however, we did not find dawn simulation superior to postawakening bright light exposure. Furthermore, they found similar responses to bright light and the dim red control. Without the exclusion of nonrelapsers, as determined in a withdrawal phase, their high placebo response rate (approximately 65%) may have impeded detection of a group difference. Alternatively, the reduced efficacy of bright light relative to dawn simulation in their study may have resulted from confinement to hypersomnic patients with standardized wake-up and treatment at 0600 hours, which is likely not individually optimal
(28) . In our study, bright light was used shortly after habitual wake-up time, which ranged from 0530 hours to 0900 hours (mean=0705, SD=1.16).
Given the approximate equivalence of naturalistic dawn simulation, high-density ionization, and bright light, the choice between them may depend on convenience and ease of compliance. In this respect, automated exposure to the dawn signal or ions during sleep has an advantage over postawakening bright light therapy. On the other hand, dawn presentation in the bedroom can disturb a sleep partner with a later wake-up time, whereas bright light therapy can be administered privately in a separate room. Negative air ionization during sleep appears to be the most innocuous alternative; thus far, we have received no reports of disturbance in bed partners. Although the antidepressant effect of negative air ionization in seasonal affective disorder recently has been independently replicated using postawakening administration (personal communication, R.K. Flory, May 23, 2006), the result for administration during sleep remains a novel observation.
For open treatment, we recommend starting patients with postawakening bright light therapy, which has seen the most extensive investigation and replication
(31) . If it is unsuccessful or proves impractical, given nonresponse, intractable side effects
(33), scheduling inconvenience, or noncompliance, dawn simulation (whether naturalistic or sigmoidal), high-density negative air ionization, and antidepressant drugs
(34) provide an armamentarium of alternate treatments.