Posttraumatic stress disorder (PTSD) is associated with increased reactivity of noradrenergic activity in response to a variety of stressors
(1) . Thus, given the role of adrenergic systems in mediating hypervigilance and cardiovascular physiologic reactivity to trauma reminders, it is possible that these symptoms would respond to antiadrenergic pharmacotherapy. However, despite longstanding enthusiasm for the use of alpha 2 agonists in PTSD, no controlled trials, to our knowledge, have been published.
We compared guanfacine to placebo in veterans with chronic PTSD who were either receiving no medication or met full criteria for PTSD, despite stable doses of psychiatric medications. We predicted that guanfacine would be superior to placebo for the treatment of PTSD symptoms.
Method
Medically healthy male and female veterans with chronic PTSD who were medication-free or receiving a stable regimen of pharmacotherapy for 2 months were recruited from Veterans Administration (VA) Medical Centers in San Francisco, Fresno, Honolulu, and Palo Alto and randomly assigned to guanfacine (N=29) versus placebo (N=34). Study protocol and a consent form were approved by the institutional review boards at the University of California, San Francisco; Stanford University; and the Fresno and Honolulu VA Medical Centers. Subjects between ages 20 and 60 were included if they met DSM-IV criteria for current PTSD. Subjects were excluded if they met criteria for alcohol or substance abuse within the past 6 months; lifetime criteria for organic mental disorder, schizophrenia, schizoaffective disorder, or bipolar disorder; history of brain disease; current systemic illness affecting CNS function; myocardial infarction in the past year; or recent use of guanfacine or clonidine.
Subjects received a 1-week single-blind placebo lead in; those with 30% or greater improvement on the Impact of Event Scale-Revised
(2) were discontinued from the trial (N=7). The remaining subjects were randomly assigned to guanfacine (0.5 mg) or placebo. Three separate random-assignment lists for subjects receiving no psychiatric medications (N=16), antidepressants only (N=21), or multiple classes of psychiatric medications (N=26) were used. Patients were seen weekly for 4 weeks and then biweekly until the end of 8 weeks of treatment. Subjects randomly assigned to guanfacine received weekly 0.5 mg increments to a target dose ranging from 1.0 mg to 3.0 mg administered before sleep. The blinded physician adjusted the dose depending on response and tolerability. Compliance was assessed by pill count. Concomitant psychosocial treatment was limited to ongoing therapy initiated at least 2 months prior to random assignment. At the end of the trial, prior to breaking the blind, subjects and blinded physicians were asked to guess the randomly assigned condition.
The primary outcomes were 1) clinician ratings of PTSD and depressive symptoms with the Clinician-Administered PTSD Scale
(3) and Hamilton Depression Rating Scale; and 2) subject ratings of PTSD, mood, and anxiety symptoms with the Impact of Event Scale–Revised, Symptom Checklist-90-Revised, Sleep Quality Index, and Quality of Life Inventory.
There were no differences in age, gender, PTSD severity, duration of illness, or comorbid depression in those randomly assigned to guanfacine versus placebo in the subjects stratified for not receiving medications, receiving antidepressants only, or receiving polypharmacy.
The primary data analysis was an intent-to-treat analysis based on linear-mixed modeling using the SAS mixed procedure, conducted separately for each outcome measure. The model predicted treatment response using treatment group as a fixed factor, time as a within-subject repeated fixed factor, and participants as a random factor with subject-specific random intercepts. Preliminary analyses examined concurrent medication-use strata as both a fixed and random factor and found no significant effects or interactions involving strata, and therefore data were collapsed across medication-use strata to improve statistical power. Comparison of adverse effects from guanfacine and placebo was tested with two-tailed Fisher’s exact test.
Results
The mean dose of guanfacine was 2.4 mg (SD=0.7). Six subjects discontinued guanfacine treatment. Three participants discontinued treatment because of side effects, one because of substance abuse relapse, one because of inconvenience of participating in research, and another for lack of efficacy. Three subjects in the placebo condition discontinued because of lack of efficacy and another when the blind was broken following an asthma attack. Drop-out rates from side effects did not differ for guanfacine and placebo (p=0.09, Fisher’s exact test).
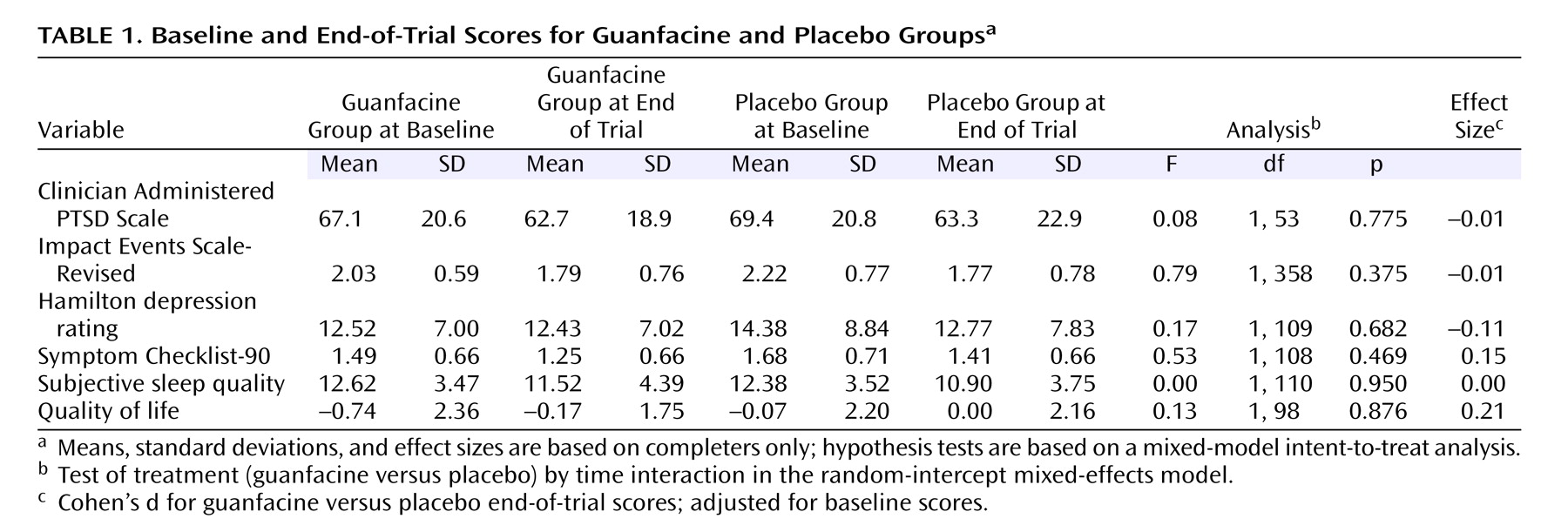
For the guanfacine group, there was a modest but significant effect of time on reducing PTSD symptoms. The mean Clinician-Administered PTSD Scale score decreased from 68.3 at baseline to 63.0 at the end of trial (F=6.86, df=1, 52, p=0.01), and the mean Impact Event Scale–Revised Total score decreased from 2.13 to 1.78 (F=20.80, df=1, 358, p<0.001). The weekly and biweekly Impact Event Scale–Revised scores in the first and second half of the trial were not different from placebo at all time points. At 8 weeks, guanfacine was not more effective than placebo for PTSD symptoms (Clinician-Administered PTSD Scale and Impact Event Scale–Revised), subjective sleep quality, general mood disturbances (Hamilton depression rating and Symptom Checklist-90), or quality of life (see
Table 1 ). There were no main or interaction effects of drug stratum on any outcome measure. The effect sizes of guanfacine treatment on both clinician and self-report PTSD scales were almost zero.
Guanfacine was associated with higher incidence of side effects, including dry mouth (59% in guanfacine group versus 15% in placebo; p<0.001, two-tailed Fisher’s exact test) and light-headedness (24% in guanfacine group versus 3% in placebo; p=0.019, Fisher’s exact test), with a tendency toward greater somnolence (48% in guanfacine group versus 23% in placebo, p=0.063, Fisher’s exact test). Guanfacine resulted in a greater drop in mean blood pressure in the guanfacine group (131.0/84.5 mm Hg at baseline versus 127.0/80.1 mm Hg at end of trial) versus placebo (133.9/85.6 mm Hg at baseline versus 133.4/84.5 mm Hg at end of trial) (F=5.39, df=1, 396, p=0.01 for systolic blood pressure; F=4.25, df=1, 397, p=0.04 for diastolic blood pressure) based on the group-by-time interaction terms in the mixed-model analysis. Notwithstanding these differences, only 59% of subjects and 56% of study clinicians correctly guessed the random assignments at the end of the trial.
Discussion
Relative to placebo, guanfacine did not result in greater improvement in PTSD symptoms, depression, general psychological distress, sleep quality, or quality of life. Guanfacine lowered blood pressure and was associated with more side effects. Treating physicians and subjects performed near chance on guessing the randomized condition.
One challenge for interpreting these results is to reconcile our negative finding with the positive results reported for prazosin
(4) . The main difference is that guanfacine lowers synaptic availability of norepinephrine for all adrenergic receptor types, whereas prazosin specifically blocks the postsynaptic alpha-1 receptor, leaving other adrenergic receptors intact. It is possible that lowering the synaptic pool of norepinephrine may produce a mix of positive and negative effects that, in balance, negate any net benefit.
We considered several other explanations for our negative finding. Three-quarters of the guanfacine group met full criteria for chronic PTSD, despite a stable regimen of pharmacological treatment. Thus, participants receiving the drug could justifiably be considered a treatment refractory group. However, our group receiving treatment was typical of patients treated in specialized PTSD clinics where alpha 2 agonists would be considered. The size of the guanfacine group has limited power; however, it is large enough for estimating effect sizes for a larger randomized controlled trial. The effect size of zero suggests no promise for demonstrating efficacy, even with larger groups. Controlled studies are needed to determine if anti-adrenergic agents with other mechanisms of action hold greater promise for the treatment of chronic PTSD.