We predicted that individuals with ADHD would make more errors of commission than typically developing healthy comparison subjects on the oddball task. Although no previous fMRI studies have investigated oddball task performance in ADHD subjects, previous fMRI studies of normal subject performance on oddball paradigms have reported activation to target stimuli in the inferior and middle frontal gyri
(15,
17 –19) as well as temporal regions (superior temporal gyrus [Brodmann’s area 22] and middle temporal gyrus) and parietal regions (inferior parietal lobules [including supramarginal gyrus] and superior parietal lobe)
(2,
15,
17 –20) . On the basis of morphological studies
(21) and functional imaging studies of response inhibition
(9 –
13) implicating these regions in ADHD, we predicted that activation might be aberrant in the frontal and striatal regions in the ADHD group. Further, since the parietal regions and frontal lobes play a critical role in sustained attention or vigilance
(22 –
24) (also known to be impaired in ADHD
[24] ), we also predicted aberrant activation in these regions.
Method
Participants
Male participants diagnosed with ADHD (N=14) and 12 typically developing male comparison subjects participated in the study after giving written informed consent and assent in accordance with Stanford Human Subjects Institutional Review Board requirements. All participants were paid $100 for participating in the study. Participants were right-handed and ranged in age from 14 to 18 years (group-matched; mean age=15.64 years, SD=1.15). Participants were predominantly of Caucasian ethnicity (80%), with the remaining participants identifying themselves as mixed-Asian (12%) and Hispanic (8%). Five participants in the ADHD group reported current use of stimulant medication; all five were subjected to an 18-hour washout of their stimulant medication prior to the scan. In addition, all participants did not have caffeine at least 2 hours before the scan, and of those reporting drug use (two in the ADHD group, one in the comparison group) and alcohol use (three in the ADHD group, six in the comparison group), a 3-week washout period for drugs (typically marijuana) and a 1-week washout period for alcohol were observed.
Typically developing comparison subjects were screened (via telephone interview with their primary caretaker and a follow-up written questionnaire) for absence of neurological, developmental, and psychiatric disorders and no family history of specific psychiatric disorders (i.e., ADHD, bipolar disorder, substance abuse/dependence, and conduct disorder/antisocial personality disorder). All individuals in the comparison group scored in the normative range (<65) on the Child Behavior Checklist
(25) .
ADHD diagnosis was determined through a structured clinical interview conducted with the primary caregiver of each participant during the initial telephone screening and was verified during the initial visit (Diagnostic Interview for Children and Adolescents–Version IV [DICA-IV] completed by primary caregiver). In addition, each primary caregiver completed the Conner ADHD/DSM-IV scale–Parent Version about their adolescent (ADHD Index Score: mean=75.5, SD=10.0). Individuals with a family history of bipolar disorder were excluded from participation. One participant in the ADHD group met diagnostic criteria for current major depression and was excluded from analyses to avoid confounds related to psychomotor retardation.
To familiarize subjects with the MRI environment and to minimize artifacts related to movement, a protocol using an MRI simulator was administered to subjects in the ADHD group. Specifically, a movie viewed by the subject during the simulated scan would cutoff for 3 seconds whenever the subject moved beyond a set criterion. Increasingly stringent movement criteria (decreasing from 3 to 1 mm) and increasing time periods (2 minutes, 5 minutes, 10 minutes) were selected until all subjects met a criterion of less than three movements exceeding 1 mm within a 10-minute period. Exclusion criteria of head movement greater than 3 mm and rotation less than 3° during fMRI scanning were established; no participants exceeded this movement threshold.
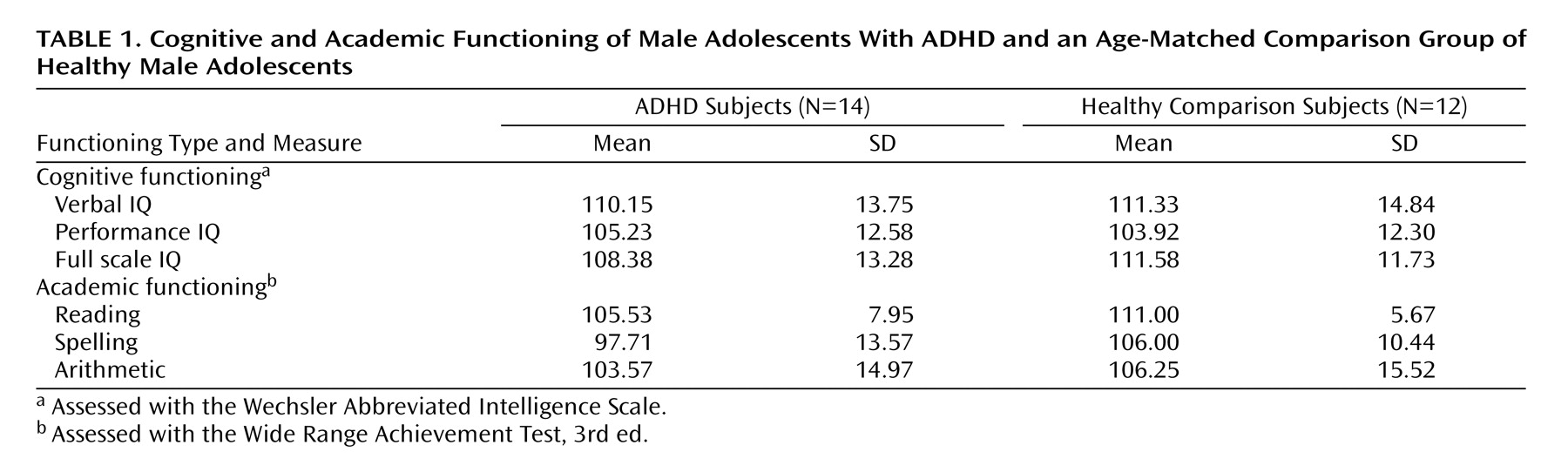
Cognitive functioning was assessed with the Wechsler Abbreviated Intelligence Scale (WAIS), and academic functioning (i.e., reading, spelling, and arithmetic) was assessed with the Wide Range Achievement Test, Third Edition (WRAT-III).
Experimental Task
In the event-related oddball task, stimuli were presented using a fast event-related design
(2,
26 –28) . A jittered stimulus presentation was used with mean inter-oddball interval of 10.9 sec (SD=9.8)
(28,
29) . Such a jittered stimulus presentation has been shown to be efficient for separating brain activation to individual events
(28) . To ensure that events could be statistically separated, we further verified that the predicted responses for each pair of stimuli were mutually orthogonal. Expected waveforms for each of the two conditions were computed after convolution with a hemodynamic response function
(30) . The correlation between expected responses to each pair of conditions was less than 0.01, allowing us to independently assess brain activation to each condition
(31) .
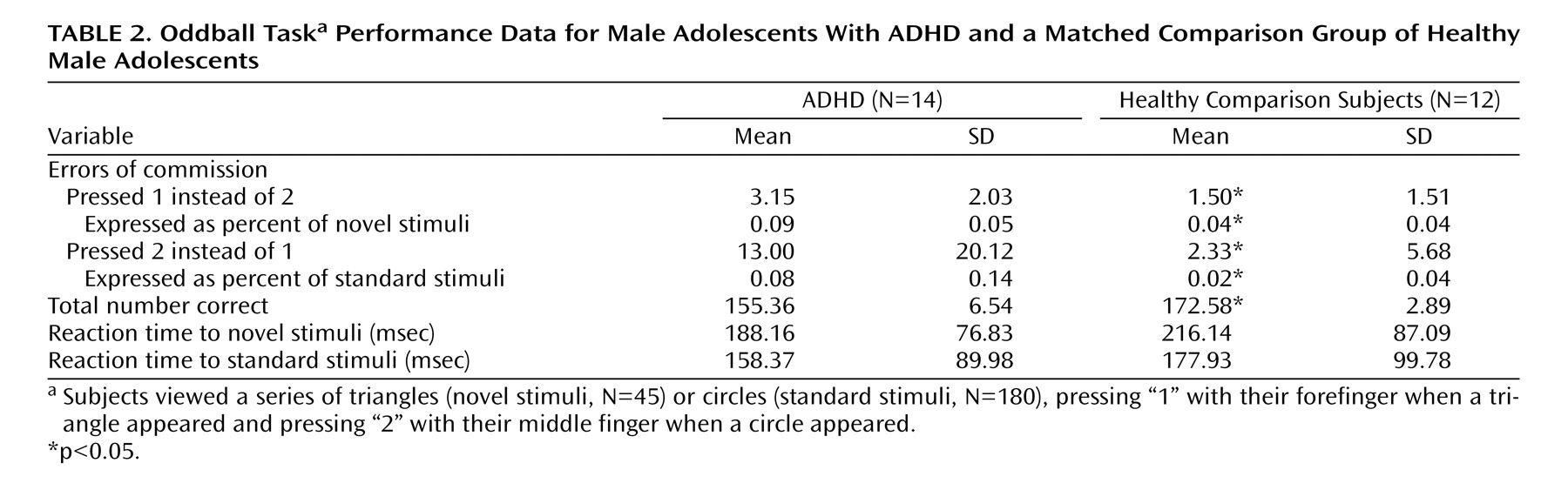
The event-related oddball experiment consisted of a 20-second rest epoch at the beginning and end of the task, during which subjects passively viewed a screen showing the word “rest.” Following the initial rest period, green circles (standard stimulus, 80% of trials) or green triangles (target or oddball stimulus, 20% of trials) were presented sequentially for 250 msec with an interstimulus interval of 1750 msec. Total number of trials was 225, which were presented in a single run. Participants were instructed to respond to the standard stimuli (circles) by pressing one button on the response box and to target stimuli (triangles) by pressing a second button on the response box. Specifically, the instructions read, “For this task, the computer will show you circles and triangles. Your job is to press 1 for triangles or press 2 for circles, as quickly as you can. Remember you want to be quick but also accurate, so do not go too fast.” Participants responded using the forefinger and middle finger of the right hand. Errors of commission (pressing 1 instead of 2/pressing 2 instead of 1) and reaction times to standard and target stimuli were recorded.
Image Acquisition
Images were acquired on a 1.5-T GE Signa scanner with echo speed gradients using a custom-built whole head coil that provides a 50% advantage in signal-to-noise ratio over that of the standard GE coil
(32) . A custom-built headholder was used to prevent head movement. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissures covering the whole brain were imaged with a temporal resolution of 2 seconds using a T2* weighted gradient echo spiral pulse sequence (TR=2000 msec, TE=30 msec, flip angle=89° and 1 interleave)
(33) . The field of view was 240 mm, and the effective in-plane spatial resolution was 3.75 mm. To aid in localization of functional data, high-resolution T1-weighted inversion recovery spoiled grass gradient recalled (SPGR) three-dimensional MRI sequence with the following parameters was used: TR=9, TE=1.9, flip angle=15°, 124 slices in coronal plane, 256×192 matrix, acquired resolution=1.5×0.9×1.2 mm. The images were reconstructed as a 124×256×256 matrix with a 1.5×0.9×0.9 mm spatial resolution.
The task was programmed using PsyScope (http://psyscope.psy.cmu.edu) on a Macintosh notebook computer. Initiation of scan and task was synchronized using a TTL pulse delivered to the scanner timing microprocessor board from a “CMU Button Box” microprocessor connected to the Macintosh. Stimuli were presented visually at the center of a screen using a custom-built magnet compatible projection system (Resonance Technology, Calif.).
Image Preprocessing
Images were reconstructed, by inverse Fourier transform, for each of the 120 time points into 64×64×18 image matrices (voxel size: 3.75×3.75×7 mm). fMRI data were preprocessed using SPM99. Images were corrected for movement using least square minimization without higher-order corrections for spin history, and normalized to Montreal Neurological Institute (MNI) template provided with SPM. Images were then resampled every 2 mm using sinc interpolation.
Statistical Analysis
Statistical analysis was performed on group data by using a random effects model
(34) along with the theory of Gaussian random fields as implemented in SPM99. This method takes advantage of multivariate regression analysis and corrects for temporal and spatial autocorrelations in the fMRI data
(35) .
Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low-frequency noise was removed with a high-pass filter (0.5 cycles/minute) applied to the fMRI time series at each voxel. A temporal smoothing function (4 mm Gaussian kernel corresponding to dispersion of 8 seconds) was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. Voxel-wise t statistics were computed using the random effects model and normalized to z scores to provide a statistical measure of activation independent of sample size. Finally, to determine the presence of significant clusters of activation, a joint expected probability distribution of height (z>1.67, p<0.05) and extent (p<0.05) threshold
(36) was used to correct for spatial correlations in the data.
For group analysis, a random effects model was used to determine voxel-wise t statistics contrasting specific events of interest (i.e., oddball versus standard stimuli). This model estimates the error variance for each condition of interest across subjects rather than across scans
(34) . The random effects model provides better generalization to the subject population, albeit with some loss in power due to averaging in the time domain and small N. This analysis proceeded in two steps. In the first step, adjusted images corresponding to the conditions/events of interest were determined. For each condition, a weighted average of the images was computed, taking into account the hemodynamic response. In the second step, these condition-specific images were contrasted in a general linear model to determine appropriate t statistics. The t statistics were normalized to z scores to determine significant clusters of activation. We examined activation to oddball versus standard events.
Neuroanatomical locations of activation were first determined using the standard Talairach atlas and then refined using the more detailed and thorough Duvernoy atlas
(37) .
Functional ROI Analysis
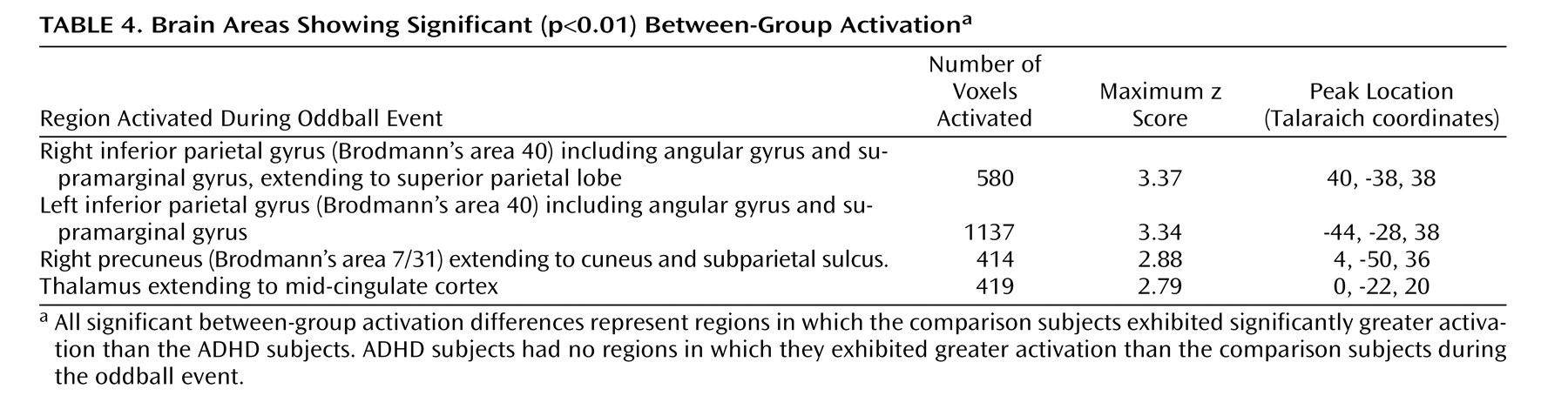
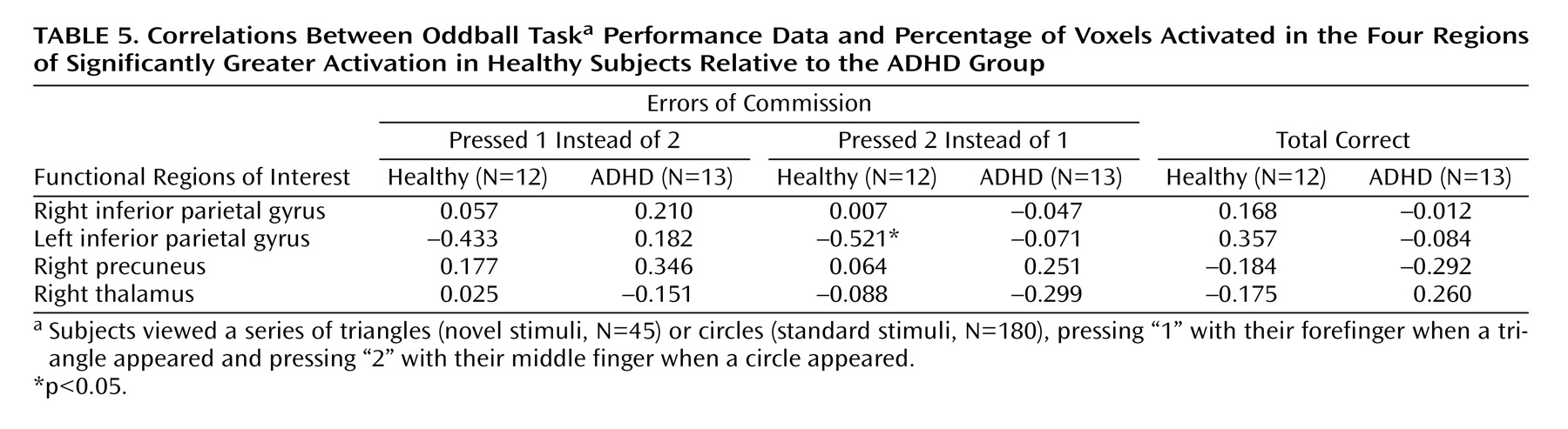
Following these analyses, exploratory analyses were conducted to assess the relationship between brain activation and performance. Specifically, regions in which the comparison subjects exhibited significantly greater activation than the ADHD group, i.e., functional regions of interest, were identified. Pearson correlations between percentage of voxels activated (height threshold: z>1.67) between the functional regions of interest and errors of commission were then computed for each group.
Discussion
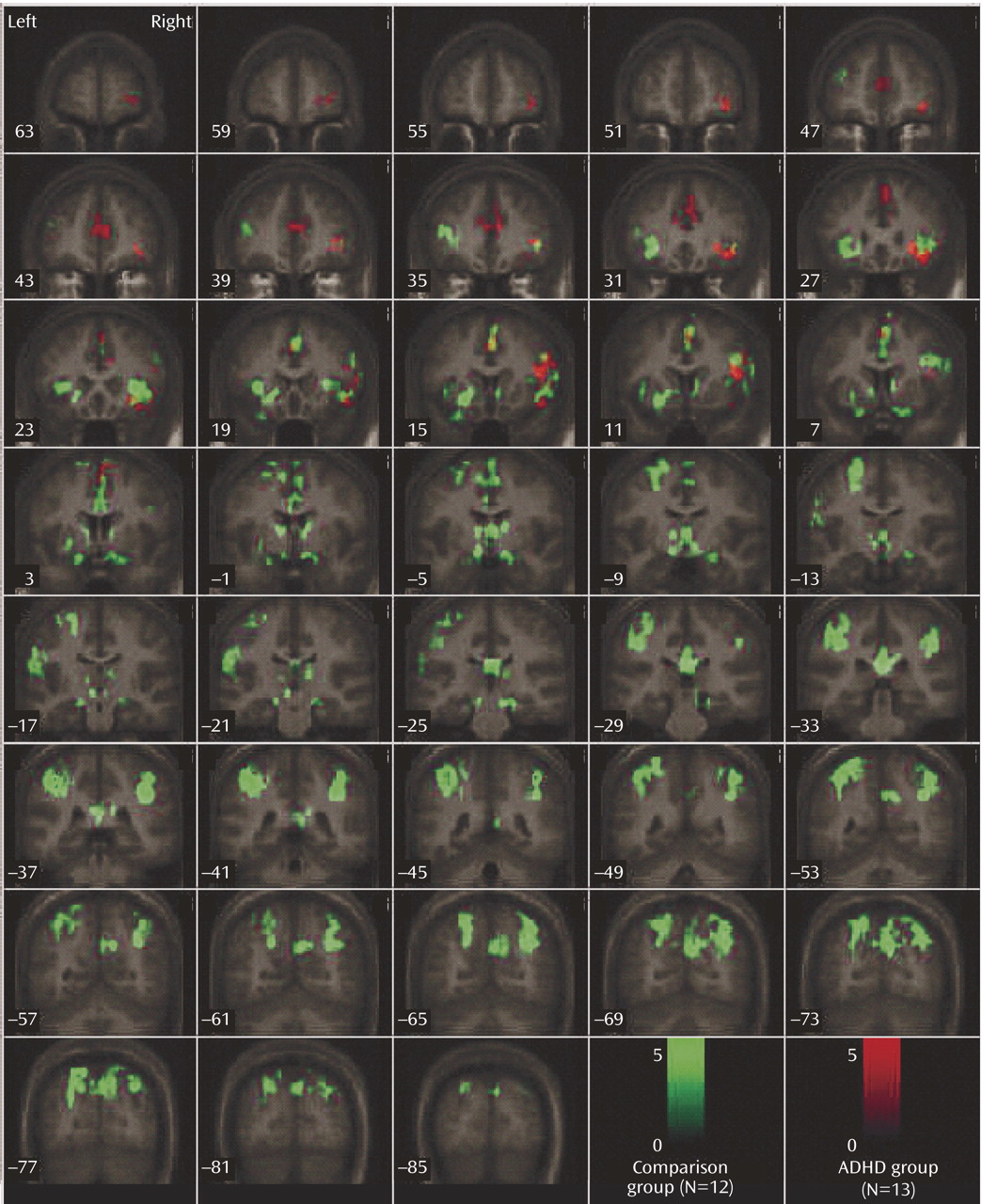
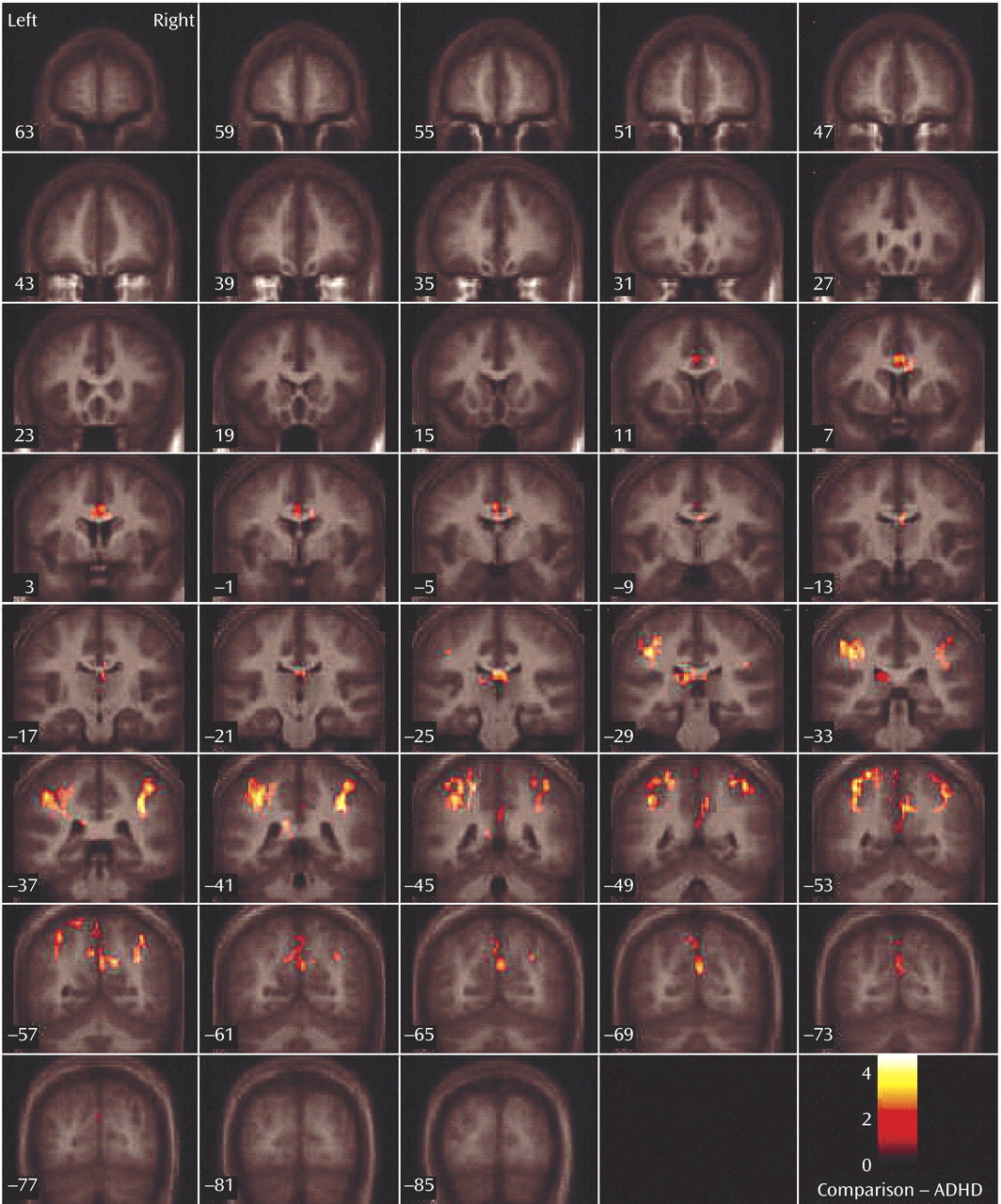
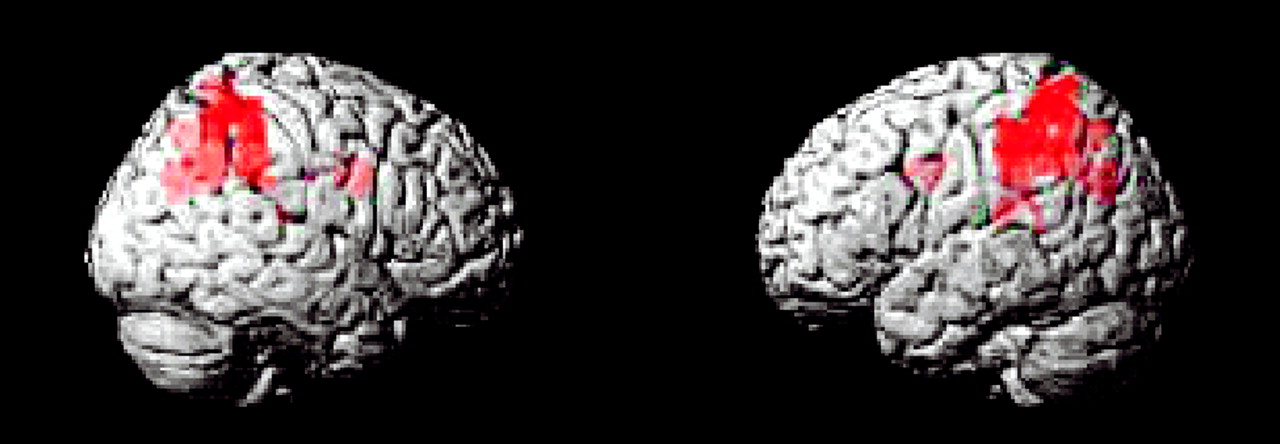
This study is the first to examine the performance and brain activation of individuals with ADHD relative to comparison subjects on an oddball task using fMRI. Results indicated significant behavioral differences between the two groups accompanied by significant differences in brain activation. Adolescents in the ADHD group made significantly more errors of commission than comparison subjects on the oddball task, but the two groups did not differ significantly on the reaction time variables. Individuals with ADHD exhibited significantly less activation than comparison subjects in the bilateral parietal association cortex, in the right precuneus, and in the thalamus. These findings suggest a critical role for these brain regions in accurate target detection and task performance, which may underlie known deficits in directed attention in individuals with ADHD.
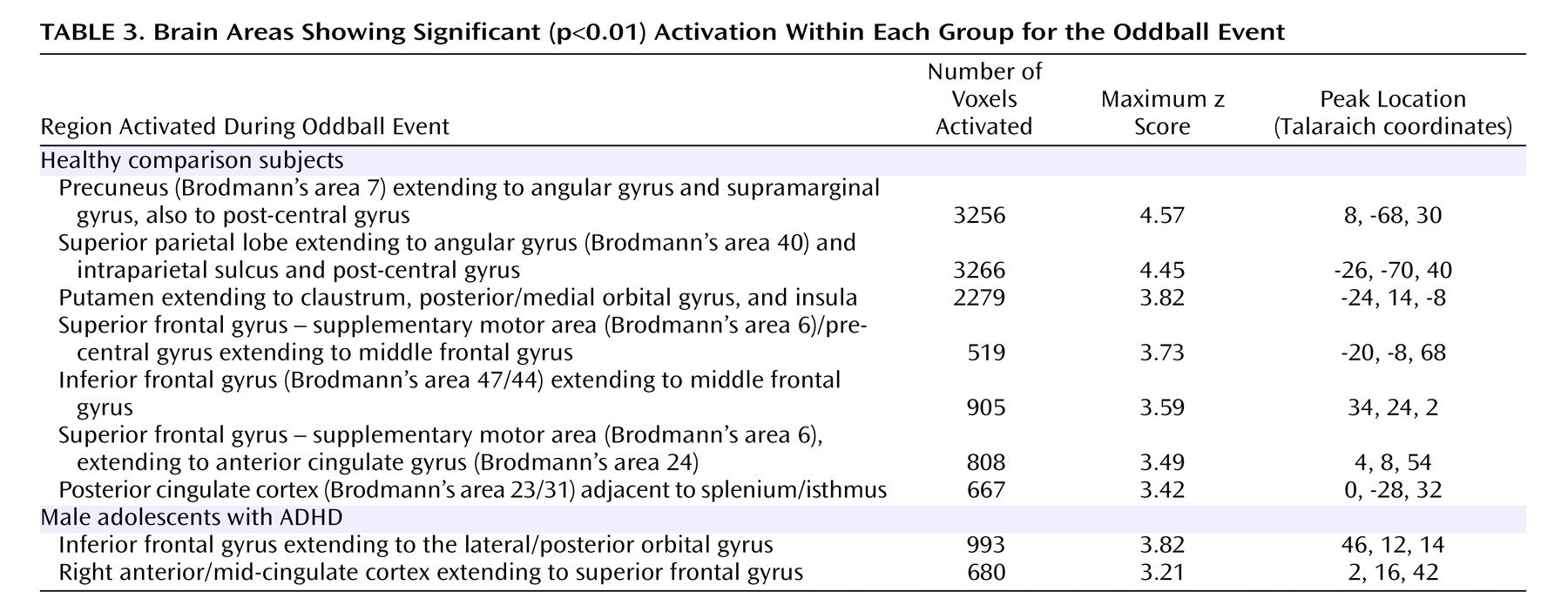
Within-group analyses revealed that the comparison group activated several regions during task performance, including peaks of activation in the bilateral parietal lobe, left putamen, bilateral superior frontal gyrus/supplementary motor area, left middle frontal gyrus, right posterior cingulate gyrus, and right inferior frontal gyrus. These regions of activation are consistent with those reported by other fMRI studies of oddball tasks in normal comparison subjects
(2,
12,
13,
15,
17 –
20,
39 –41) . It is probable that activation in the supplementary motor area reflects higher-order motor planning
(14,
42,
43) and potentially attentional shifting
(43) . Activation in the parietal lobes, cingulate, and frontal lobes is likely associated with the process of target detection/directed attention (including activation related to response inhibition, vigilance, and working memory), since these regions are consistently activated in visual oddball tasks
(19,
44) .
More specifically, anterior cingulate activation to oddball stimuli observed in the comparison group likely reflects response conflict (i.e., simultaneous activation of incompatible response tendencies
[14] ), and inferior frontal gyrus activation is thought to be related to the need to “stop” prepotent motor responses
(26,
45,
46) . Putamen activation during target detection in normal subjects has been reported previously
(47) and may be associated with response selection during controlled processing
(48) and response inhibition
(9) . Overall, those regions in which comparison subjects showed significant activation to oddball stimuli in our study are similar to those reported by target detection studies, suggesting that the task used in this study captured the construct of directed attention.
In contrast to the comparison group, individuals with ADHD showed fewer peaks of activation. Within-group analyses revealed activation in the right inferior frontal gyrus and in the right cingulate gyrus extending to the superior frontal gyrus. Again, activation in the right inferior frontal gyrus likely reflects response inhibition
(26,
45,
46), and activation in the cingulate gyrus likely reflects response conflict
(14) . Notably, activation in the cingulate region was more anterior in the ADHD group than that observed in the comparison group. It has been suggested that the cingulate can be subdivided in terms of function into the caudal cingulate zone, the rostral cingulate zone, and the anterior rostral cingulate zone
(49), with the anterior rostral cingulate zone thought to be an “error-sensitive” region
(14) . Because individuals in the ADHD group made significantly more errors of commission than comparison subjects, activation in this region may reflect response to errors or increased conflict resulting in errors of commission
(14) . However, to assess the validity of this hypothesis, continued investigation is warranted. It was not possible to investigate brain activation specifically related to errors of commission because of insufficient power.
Adolescents with ADHD did not show more activation than comparison subjects in any region on the between-group analyses. However, the ADHD group did make significantly more errors of commission and showed significantly less activation in several posterior brain regions. Exploratory functional regions of interest analyses indicated that activation in the left parietal lobe was significantly negatively correlated with errors for the comparison group, supporting the hypothesis that for comparison subjects, the left parietal cortex may be directly involved in improving behavioral performance. The fact that no significant correlations were observed between errors of commission and other regions with group differences suggests that the right parietal cortex and right cingulate are either related to performance indirectly or via a mechanism not measured in this study. Regardless, it does appear that activation in these regions plays a role in task performance (i.e., target detection) and may be associated with improving performance in the comparison group.
Male adolescents with ADHD activated the bilateral superior parietal lobe, angular and supramarginal gyri, the right precuneus, and the thalamus significantly less than comparison subjects. There is strong evidence to suggest that activation in the superior and inferior parietal lobes is related to sustained attention/vigilance and attentional or set shifting
(50,
51), or more generally, effortful attention
(52,
53) . Similarly, the precuneus has been shown to activate in response to relevancy and context
(54,
55), as well as attentional shifting
(43,
51,
56,
57) . The majority of fMRI studies investigating a number of oddball paradigm variants report activation in the bilateral supramarginal gyrus (most typically in Brodmann’s area 40), which suggests a critical role for this region in target detection
(2,
15,
19 –
20,
58) . Other studies suggest a role for the left parietal cortex, and in particular the supramarginal gyrus, in motor attention
(59,
60), and the right supramarginal gyrus in attentional shifting
(61) . Thus, the confluence of evidence suggests that the bilateral supramarginal gyrus plays a role in postsensory processing of salient stimuli for evaluation, categorization, response, and decision making
(2) . It is interesting that the pattern of results for the ADHD group is consistent with impairments in the regions thought to be associated with goal-directed attention (i.e., a top-down process for attention to features of a stimulus) including the fronto-parietal network and in particular the posterior parietal region, which activates in response to attention switched between objects presented in the same location [e.g., the oddball stimuli
(53) ].
Group differences in angular gyrus activation were somewhat unexpected, since this region is most frequently reported as involved in language
(62) and number processing
(63,
64) . However, angular gyrus activation also has been observed in response to visuospatial functioning
(65), visual pattern recognition
(66), and perception of spatial features of objects
(67), which are clearly relevant for the visual oddball task. Activation in the thalamus has been reported in previous oddball studies
(2,
17,
18,
41) and is thought to reflect engagement of the hippocampal-hypothalamic network in mediating the initial orienting response
(2,
3) .
Failure of the ADHD group to activate to the same degree as comparison subjects in parietal/precuneus regions suggests that they had difficulties in shifting attention and alternating motor responses to target stimuli, possibly one reason why this group made more errors than comparison subjects. A hypothesis that individuals with ADHD have impaired attention shifting is supported by our finding that this group made more of both errors of commission types. That is, they appeared to have trouble inhibiting a prepotent response and shifting to make an alternative response to target stimuli (i.e., pressing 1 instead of 2), but they also had difficulty shifting back to making the prepotent response (i.e., pressing 2 instead of 1). Thus, the difficulties expressed by individuals with ADHD appear to reflect more than impaired response inhibition; they also reflect fundamental impairments in the ability to shift attention and make alternative responses. In addition, errors of commission signal impulsivity, which also is indicative of impaired directed attention in ADHD.
Limitations of this study include the relatively small study group size. In addition, despite the fact that we excluded one subject who met criteria for current major depression, a small percentage of individuals in the ADHD group met diagnostic criteria for other disorders, which may have confounded the findings. It should be noted that interpretation of fMRI group differences may reflect only performance impairments, as opposed to a deficit specific to diagnosis with ADHD. Future studies with sufficient power and errors to conduct a covariance analysis with error rates will be needed to disentangle this important question.
Overall, the results of this study demonstrate a relationship between impaired target detection and brain function in ADHD; more generally, these results contribute to our understanding of the neural correlates of impaired ability to direct attention in ADHD. Our findings suggest that individuals with ADHD have significant aberrations in the posterior parietal attentional system, which is known to play a critical role in maintaining and shifting attention. Prior imaging studies of ADHD have primarily focused on the role of frontal and striatal regions in executive functions, such as response inhibition
(9 –
11), since morphological abnormalities of these regions are commonly reported
(21) . Our results, however, suggest that it is important to reconsider the notion of ADHD as primarily a disorder of frontal-striatal function, and consider the role of the parietal attentional system in the behavioral phenotype of ADHD. Additional studies are warranted to investigate the relationship and interconnections between frontal, striatal, and parietal regions in executive dysfunction in ADHD.