Growing evidence suggests that obsessive-compulsive disorder (OCD), trichotillomania (compulsive hair pulling), and other conditions may represent an obsessive-compulsive spectrum or family of disorders
(1) . The symptoms of OCD and trichotillomania suggest problems inhibiting motor behavior (impulsivity). However, OCD is also characterized by repetitive mental acts or behaviors performed according to rigid rules (DSM-IV). It has been proposed that these compulsive symptoms may be mediated by problems with cognitive flexibility, such as the ability to shift attentional focus
(2) . We compared motor inhibition and cognitive flexibility in subjects with OCD and trichotillomania using two well-validated neurocognitive tasks. Motor inhibition was assessed with the Stop-Signal Task
(3), which provides a sensitive estimate of the time taken to internally suppress motor responses. This task has been shown to be sensitive to motor impulsivity associated with attention deficit hyperactivity disorder (ADHD) and damage to the right inferior frontal gyrus
(4,
5) . Cognitive flexibility was assessed with the Intradimensional/Extradimensional Shift Task, developed from the Wisconsin Card Sorting Test of frontal lobe integrity
(6) . The Intradimensional/Extradimensional Shift Task examines different components of attentional flexibility, including reversal learning, set formation, and the ability to shift attention between stimulus dimensions
(7) . We predicted that subjects with OCD would show deficits in motor inhibition and cognitive flexibility but that subjects with trichotillomania would show deficits in motor inhibition only.
Method
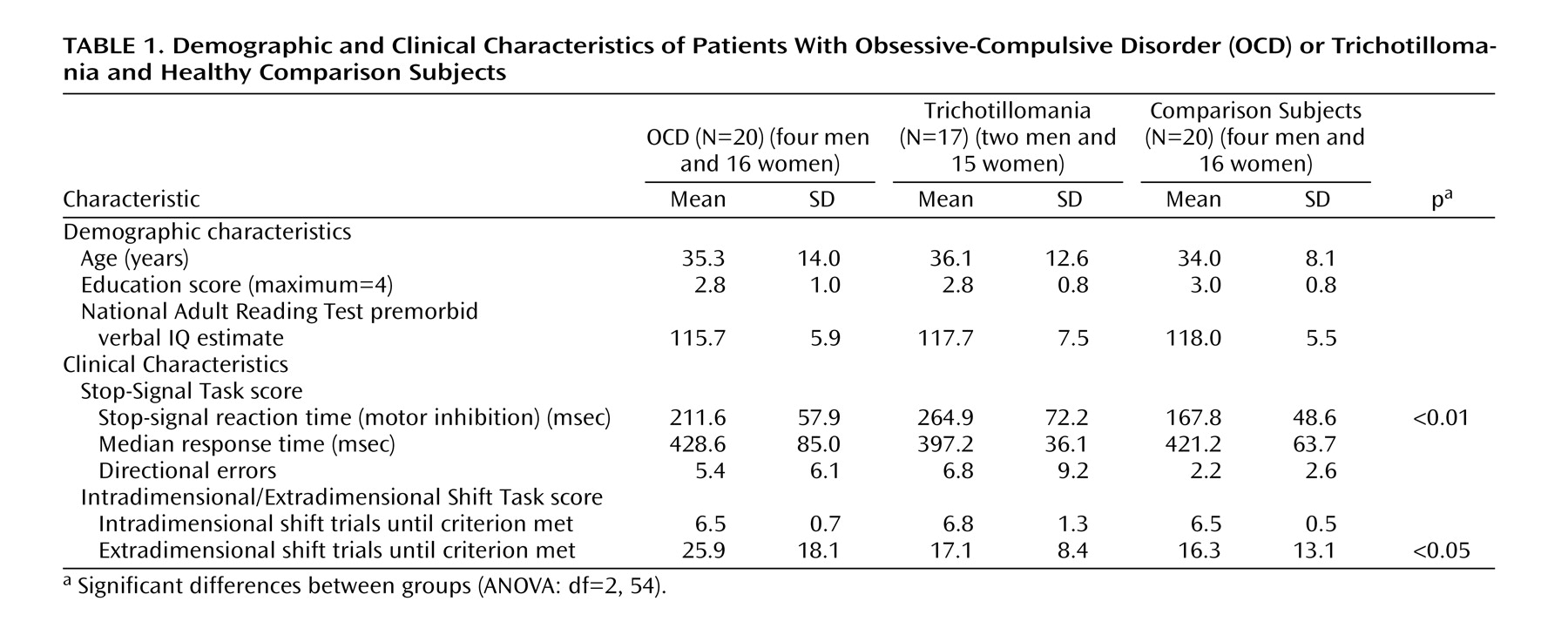
Twenty OCD patients, 17 trichotillomania patients, and 20 healthy comparison subjects gave written informed consent and were administered the tasks. The groups were matched for age, education, and IQ
(8) (
Table 1 ) (analysis of variance [ANOVA], all p>0.10). DSM-IV diagnoses were made by a fully certified consultant psychiatrist using an extended clinical interview supplemented by the Mini International Neuropsychiatric Interview
(9) . For OCD, only archetypal illness involving contamination or checking was allowed (no hoarding). The participants were excluded if they had significant comorbidities, including major depression (Montgomery-Åsberg Depression Rating Scale score [MADRS] >16)
(10), Tourette’s syndrome, psychosis, eating disorders, and other anxiety disorders. OCD severity was assessed with the Yale-Brown Obsessive Compulsive Scale
(11) . The patients with OCD scored >16 on the scale; 16 of 20 were receiving selective serotonin reuptake inhibitors (SSRIs), and the remainder were unmedicated (free from psychotropic medications for at least 6 months before cognitive testing). The severity of trichotillomania was assessed with the Massachusetts General Hospital Hairpulling Scale
(12), and patients with trichotillomania were unmedicated (free from psychotropic medications for at least 6 months before cognitive testing).
On the computerized Stop-Signal Task, subjects respond rapidly to left- or right-facing arrows on a computer screen with corresponding motor responses and attempt to inhibit responses when an auditory “stop signal” sounds. With a tracking algorithm, this task estimates the time taken to internally suppress prepotent motor responses (stop-signal reaction time)
(3,
5) .
The Intradimensional/Extradimensional Shift Task
(2) is a nine-stage visual discrimination task with multidimensional stimuli. Two stimuli are displayed at a time, and feedback is provided so that the subject can learn which stimulus is correct. To pass each stage, six consecutive correct responses are required within 50 trials; otherwise, the task ends. The rule for correct responding is modified at the start of each task stage in order to dissociate different aspects of cognitive flexibility
(7) . For example, the intradimensional shift stage examines rule generalization when novel stimuli are introduced, whereas the extradimensional shift stage examines the ability to inhibit or shift attention away from previously relevant stimulus dimensions (akin to a category shift on the Wisconsin Card Sorting Test). The subjects who failed to pass a stage were assigned a score of 50 trials to the criterion for that stage and were excluded from data analysis for subsequent stages not attempted.
To detect overall group differences for demographic and cognitive measures, ANOVA was employed. In the instances in which significant group differences were found with ANOVA, exploratory pairwise analyses were conducted with Fisher’s least significant difference tests as appropriate.
Results
The mean Yale-Brown Obsessive Compulsive Scale score for the OCD group was 20.4 (SD=4.1). The mean Massachusetts General Hospital Hairpulling Scale score for the trichotillomania group was 16.4 (SD=4.7). MADRS scores for all groups were beneath the cutoff point for clinically significant depression (<10)
(13) —OCD patients: mean=6.9 (SD=4.4), trichotillomania patients: mean=4.2 (SD=3.6), healthy comparison subjects: mean=3.1 (SD=4.4). There was a significant overall group difference on MADRS scores (ANOVA: F=8.44, df=2, 54, p<0.01). Post hoc least significant difference tests showed that the subjects with OCD had significantly higher MADRS scores than other groups (OCD group versus trichotillomania group: p<0.05; OCD group versus healthy comparison group: p<0.01), whereas the scores of the trichotillomania and healthy comparison groups did not differ on the MADRS (p>0.10).
On the Stop-Signal Task, the groups differed significantly on stop-signal reaction times (F=12.19, df=2, 54, p<0.01). Post hoc analysis revealed that the patients with trichotillomania had longer stop-signal reaction times than the OCD patients, who had longer reaction times than the healthy comparison subjects (trichotillomania group versus OCD group: p<0.01; trichotillomania group versus healthy comparison group: p<0.001; OCD group versus healthy comparison group: p<0.05). The groups did not differ significantly in the number of directional errors overall (p>0.05) or on median “go” response times (p>0.10).
In terms of the number of trials taken to reach the criteria for the intradimensional shift and extradimensional shift stages, there was a significant interaction of stage and group (F=3.59, df=2, 54, p<0.05). The groups differed from each other only at the extradimensional shift stage (F=3.44, df=2, 54, p<0.05), with post hoc analysis revealing that the OCD group required more trials at the extradimensional shift stage compared to both of the other groups (OCD group versus trichotillomania group: p<0.05; OCD group versus healthy comparison group: p<0.05). The trichotillomania and healthy comparison subjects did not differ at the extradimensional shift stage (all p>0.10). For other stages of the task (including those necessitating rule reversals), the performance of the OCD, trichotillomania, and healthy comparison groups did not differ significantly (ANOVAs: all p>0.10).
Analyses were undertaken with Pearson’s correlations. Symptom severity in trichotillomania (Massachusetts General Hospital Hairpulling Scale scores) correlated significantly with stop-signal reaction times (r=0.564, p<0.02). Symptom severity in OCD (measured with the Yale-Brown Obsessive-Compulsive Scale) did not correlate with any task measures (p>0.10). MADRS scores did not correlate significantly with performance on either task within the clinical groups (all p>0.10).
Discussion
The clinical phenotypes of OCD and trichotillomania suggest an overlap in terms of impulsivity or problems inhibiting motor behavior. However, only OCD is associated with rigid mental acts or behaviors that imply problems with cognitive flexibility
(2) . Obtained with objective measures, our results provide direct support for these contentions: both clinical groups showed severely impaired motor inhibition (the Stop-Signal Task), but only the OCD group showed a deficit in cognitive flexibility (with the Intradimension/Extradimensional Shift Task). Impairment of motor inhibition correlated significantly with symptom severity in trichotillomania. In OCD, the cognitive flexibility deficit was specific to the stage in which it was necessary to inhibit or shift attentional focus away from a previously relevant stimulus dimension. Individual scores for unmedicated OCD patients were comparable to medicated patients (within 0.5 SDs of the mean for the medicated subgroup on key task measures).
This study is the first to our knowledge to demonstrate motor impulsivity across obsessive-compulsive symptom disorders, with deficits in cognitive flexibility limited to OCD. This implies distinct but also overlapping neurobiological underpinnings to disorders whose nosological status is currently under review. Limitations of this study include the group size and the possible confounding effect of SSRI medications in the OCD group. Future research should expand on the concepts of impulsivity and compulsivity, examine the effects of pharmacological and psychological treatments on behavioral inhibition, and delineate the contribution of frontal-striatal circuitry and neurochemical modulation of these processes. This will be relevant to the treatment of impulsive as well as compulsive aspects of the obsessive-compulsive symptom disorder profile.