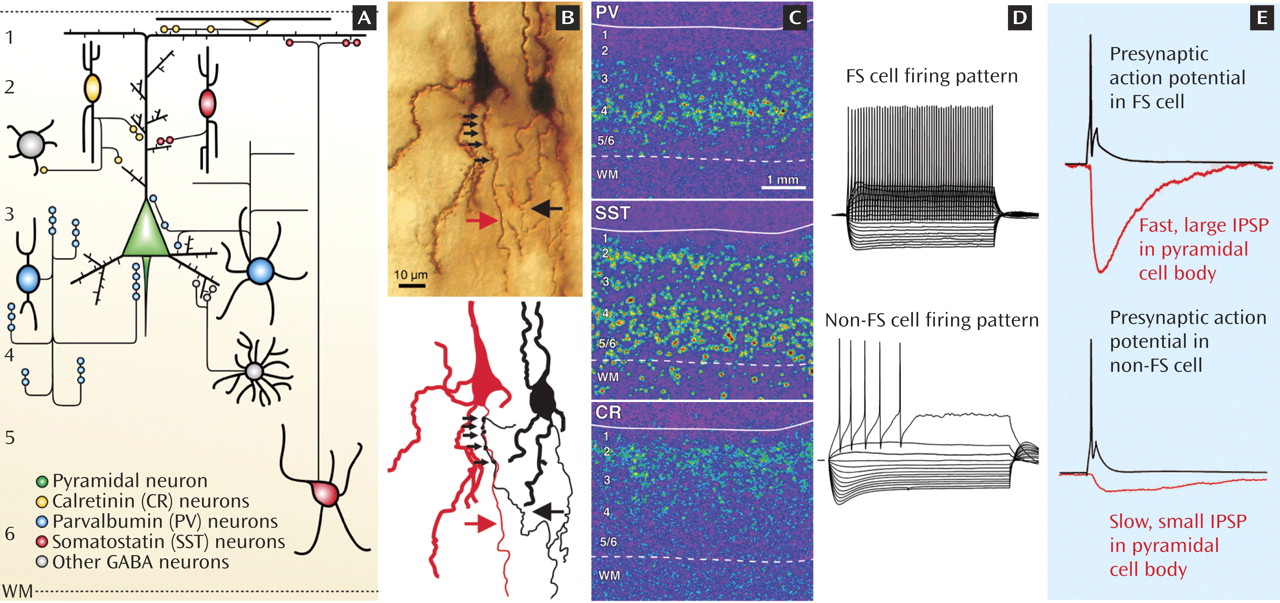
Excitatory pyramidal cells convey to other brain regions the neural signals generated by the local circuits in each cortical area. Pyramidal cell activity is strongly modulated by interneurons that utilize the inhibitory neurotransmitter GABA. Subclasses of cortical interneurons are defined by several characteristics. Biochemically, some interneurons express the calcium-binding proteins parvalbumin (PV) or calretinin (CR), whereas others contain the neuropeptide somatostatin (SST). Morphologically, the axons of SST neurons target pyramidal cell dendrites, whereas the GABA synapses formed by PV neurons are found at or close to pyramidal cell bodies (panel A). For example, as shown in panel B, the axons of PV-containing chandelier neurons (black arrow) make multiple synaptic contacts (small arrows) at the pyramidal cell axon initial segment (red arrow). Anatomically, these three subclasses of interneurons have differential distributions across the six layers of the neocortex, as illustrated by the distinctive expression patterns of their messenger RNAs (panel C). Electrophysiologically, PV neurons are called fast-spiking (FS), since during sustained stimulation they fire fast action potentials at a constant interval (panel D). In contrast, non-FS neurons, such as the SST neurons, fire slower action potentials at progressively longer intervals.
In interneurons, the discharge of an action potential (black traces in panel E) triggers synaptic GABA release, which activates GABA-A receptors in the targeted pyramidal cell and produces an inhibitory postsynaptic potential (IPSP). The IPSPs generated by FS/PV neurons have a large amplitude and fast time course (top red trace), reflecting the perisomatic location of the synaptic contacts. In contrast, IPSPs generated by dendrite-targeting non-FS/SST neurons are small and slow at the pyramidal cell body (bottom red trace), indicating that their physiological inhibitory effect is strongest in the dendrites.
Interneuron-mediated
inhibition does not simply constrain pyramidal cell excitation but precisely controls the timing of pyramidal cell firing, causing large groups of nearby pyramidal cells to oscillate between active and silent states. GABA neuron activity induces rhythms of different frequencies, with each oscillation frequency apparently involving different interneuron subclasses. The synaptic signaling by cell body-targeting PV neurons and by dendritic-targeting SST neurons appears to be impaired in schizophrenia, suggesting that the alterations in circuit oscillations and neuronal synchrony observed in the illness are due, at least in part, to selective disturbances in GABA transmission mediated by these interneuron subtypes. Because neuronal synchrony increases the efficacy of synaptic communication within and between cortical areas, interneuron alterations in schizophrenia are likely to have substantial effects on cognitive processes that are dependent on the integration of neural signals across the neocortex.