Catechol-
O -methyltransferase (COMT) catalyzes the first step in a major degradation pathway of the catecholamine neurotransmitters, including dopamine. The COMT gene on chromosome 22q11 contains a functional polymorphism (
Val 108/158 Met, rs4680) that affects the enzyme’s temperature sensitivity.
Met alleles result in a fourfold decrease in enzyme activity at body temperature
(2) leading to slower inactivation of released dopamine within the brain, notably in the prefrontal cortex where COMT is responsible for 60% of dopamine degradation
(3) . As a result, COMT knockout mice show a threefold increase in concentrations of dopamine in the prefrontal cortex but not in other areas with strong dopaminergic connections, such as the striatum
(4) .
Prefrontal dopaminergic dysfunction is a widely accepted feature of schizophrenia
(5) . Many studies have now assessed the association between the
Val 158/108 Met polymorphism and schizophrenia
(6) . One recent meta-analysis found
Val to be a small but reliable risk factor for schizophrenia in people of European ancestry
(7), although others have not supported this result
(8) .
Regardless of any association with schizophrenia, the
Val allele is common among the general population
(9) in which it affects cognitive functions that rely on the prefrontal cortex. Healthy adults with the
Val allele perform more poorly on cardinal tests of executive function such as the Wisconsin Card Sorting Test than those with
Met alleles
(6,
10,
11) . Some observational studies have failed to replicate the association
(12,
13), but experimental studies showing that COMT inhibitors improve working memory in rats
(14) and humans
(15) offer potent support.
Functional changes also occur in the frontal cortex during adolescence. While the majority of executive functions become operational at around age 8
(20), many continue to develop until early adulthood
(21) . Moreover, functional MRI studies show that children show activation in alternative neuronal circuits
(22) or more widespread areas
(23) of the prefrontal cortex when compared with adults performing the same tasks. If the frontal lobes continue to mature during adolescence, the cognitive functions performed by prefrontal cortex circuitry in adults may be performed by different or more diffuse circuits in prepubertal children. If the effect of COMT genotype is particularly important in the prefrontal cortex, then COMT genotype may have little effect on prepubertal cognitive performance.
Large studies with specific neuropsychological measures are needed to determine the cognitive, or other, effects of candidate genes for psychiatric disorders in the general population. These have the potential to map genetic contributions to both normal and abnormal development and to detect what are likely to be small effects of individual variants. We assessed the effect of the COMT Val 158/108 Met polymorphism on cognitive function in a large birth cohort. We hypothesized that effects of the COMT genotype on cognition would be found in tasks that rely on frontal lobe function and that the magnitude of effect would be larger in children who were further through puberty.
Results
Data Availability
Val 108/158 Met genotype was available for 8,707 children from the cohort ( Val / Val =2,126; Val / Met =4,294; Met/Met =2,287). The overall frequency of the Met allele was 50.9%. This distribution was consistent with Hardy-Weinberg equilibrium (χ 2 =1.55, df=1, p>0.10).
Pubertal report was available for 4,821 of these children. The majority of children of both genders were in Tanner stage 1 (boys=83.4%; girls=81.5%), with a few girls (3.2%) and almost no boys (<0.01%) in stages 3 or above.
Biases were present in the availability of genotype data. Children for whom parental permission was granted to undertake genotyping scored more highly than the remainder on verbal IQ (t=–4.22, df=6991, p<0.001), performance IQ (t=–2.12, df=6514, p<0.05), and total IQ (t=–3.50, df=6454, p<0.001). They did not differ on any of the measures of executive function. There were no differences in cognitive performance between children who were successfully genotyped and those where genotyping failed.
There was no effect of gender on Val 158/108 Met genotype (χ 2 =0.27, df=2, p=0.87). There were no associations between pubertal development and COMT genotype in girls (χ 2 =0.91, df=2, p=0.63) or boys (χ 2 =1.83, df=2, p=0.40).
Ethnicity was known for 8,602 children. Only 401 children (4.7%) were described as having a nonwhite ethnic background, which we refer to as black and minority ethnic. Met alleles were underrepresented among the black and minority ethnic children (42.0%) relative to the reference group (51.4%) (χ 2 =27.0, df=1, p<0.001). Pubertal data availability was also lower for the black and minority ethnic children (girls χ 2 =28.1, df=1, p<0.001; boys χ 2 =32.4, df=1, p<0.001). Because of these biases, and to increase the ethnic homogeneity of the cohort, we excluded the black and minority ethnic children from all subsequent analyses.
Comparison of COMT Genotypes
One-way ANOVA was used to compare mean cognitive scores between genotypes across the cohort. Three cognitive measures showed an effect of genotype. Working memory count span was significantly associated with increasing Met alleles (F=5.166, df=2, 5046, p<0.05), and the same effect was seen in the global score (F=3.371, df=2, 5046, p≤0.05). In addition, verbal IQ showed an improvement of 0.8 IQ points for each Met allele (F=3.452, df=2, 5342, p<0.05). Post hoc comparisons of performance on each WISC subtest by genotype revealed no significant differences on any individual test.
COMT and Gender
The second analyses included gender, genotype, and gender-by-genotype interaction terms. There was a large effect of gender on almost every measure except total IQ scores, such that girls outperformed boys on all measures except block design and verbal IQ.
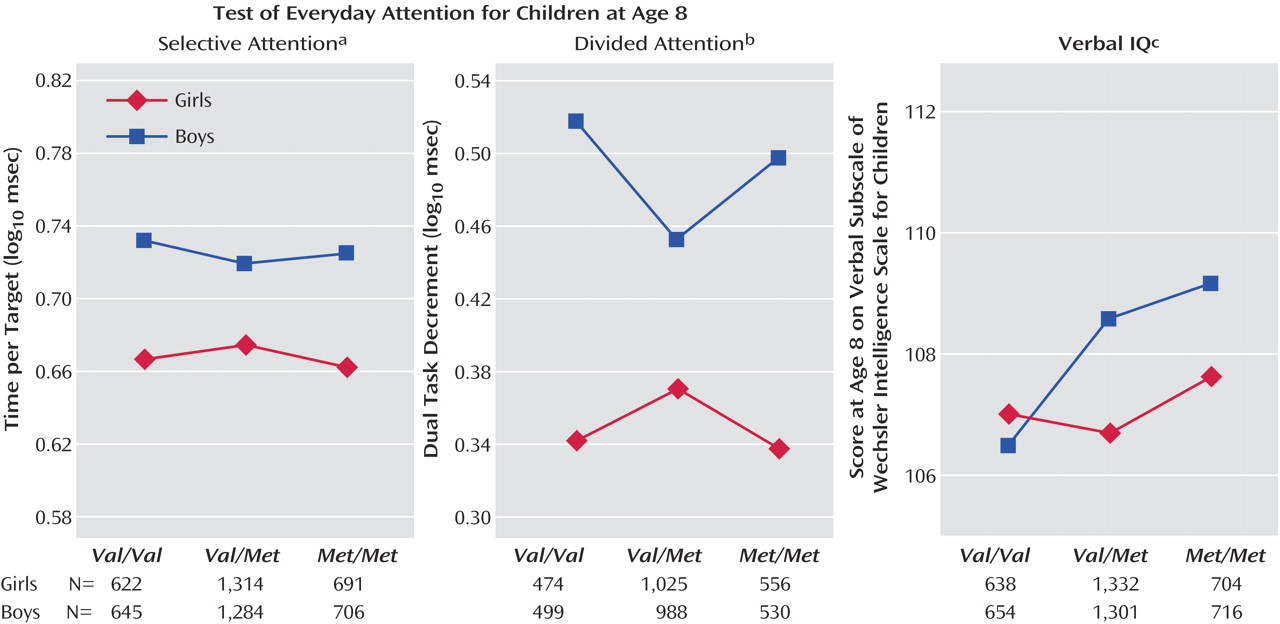
As seen in
Figure 1, there was a significant gender-by-genotype interaction for selective attention, and gender-by-genotype interactions approached significance for divided attention and verbal IQ (the gender-by-genotype interaction also approached significance for total IQ [F=2.66, df=2, 4947, p<0.10]). The IQ effects were such that boys showed a larger effect of genotype on cognition, however in selective and divided attention the interaction was nonlinear; heterozygotes appeared to be at an advantage in boys but a disadvantage in girls (
Figure 1 ).
Significant main effects of genotype were found for working memory count span (F=5.24, df=2, 5043, p<0.01) and global score (F=3.44, df=2, 5043, p<0.05). There was also a main effect of genotype on verbal IQ (F=3.42, df=2, 5339, p<0.05) and a tendency toward an effect of genotype on the Opposite Worlds task (F=2.42, df=2, 5276, p<0.10). Inspection of the data showed that the effect on IQ was not caused by outliers; rather, in boys there appeared to be a population shift toward higher verbal IQ with increasing Met allele dose.
COMT and Puberty
The final stage of analyses assessed main effects of genotype and pubertal stage, and a genotype-by-puberty interaction term, separately for girls and boys.
In girls, models comprising these terms did not fit the data adequately, implying no significant effects on cognition of either genotype or pubertal stage. The only exception to this was selective attention, where puberty showed some effect on performance (F=3.80, df=1, 2050, p=0.05).
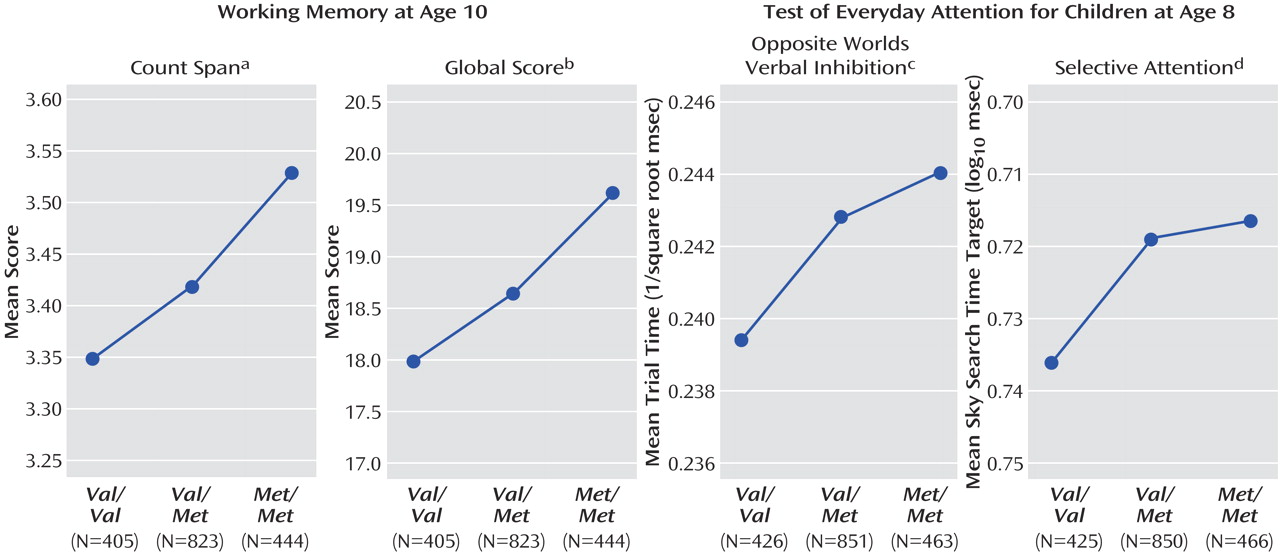
In boys, there were significant modeled effects in six of the cognitive measures. There was a main effect of genotype on verbal IQ (F=9.97, df=2, 1763, p<0.001) and total IQ (F=4.28, df=2, 1639, p<0.05), working memory count span (F=3.09, df=2, 1666, p<0.05) and global score (F=3.86, df=2, 1666, p<0.05) and the Opposite Worlds verbal inhibition task (F=3.02, df=2, 1734, p<0.05; see
Figure 2 ). In addition, there was a nearly significant effect of genotype on selective attention (F=2.91, df=2,1735, p=0.05). In all cases effects were in the predicted direction, with improved performance in children with one or more
Met alleles relative to those with two
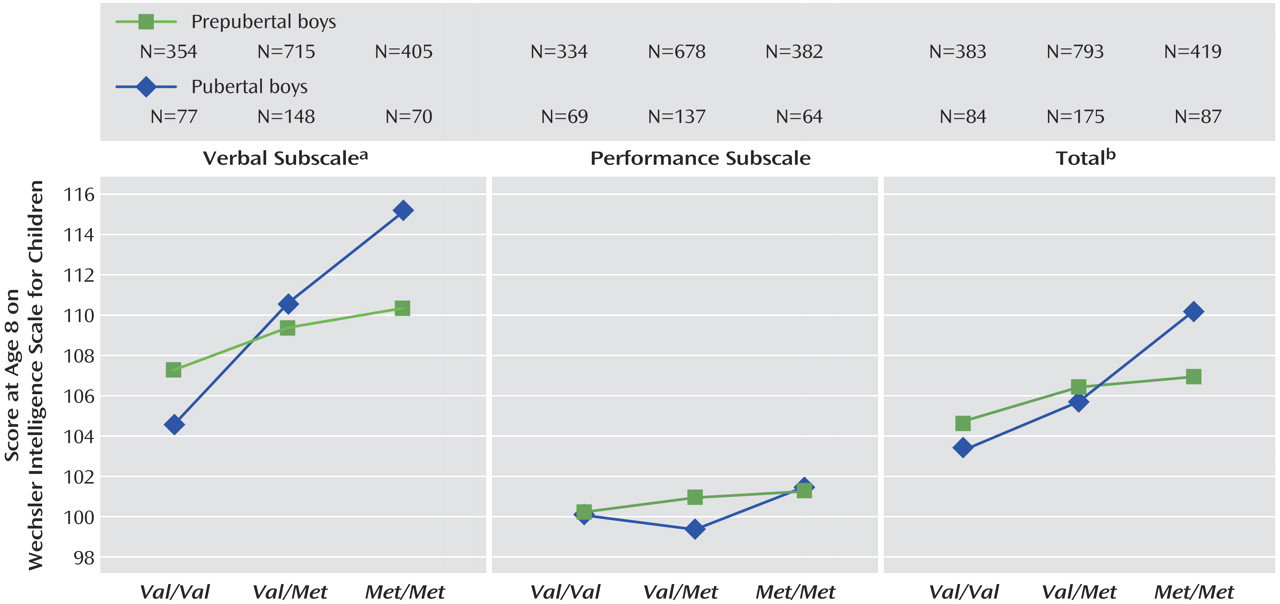
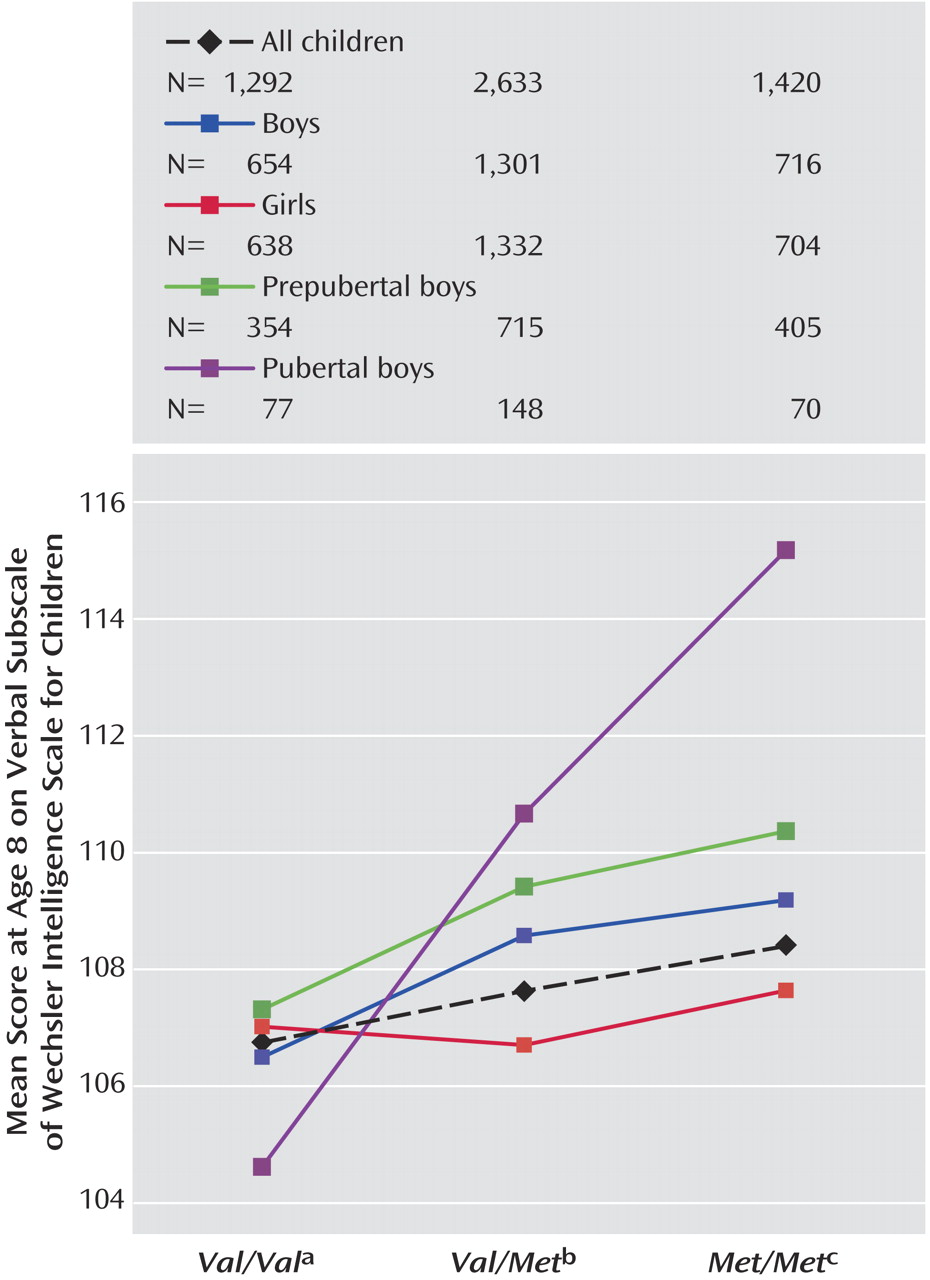
Val alleles. For verbal IQ, there was a genotype-by puberty interaction effect (
Figure 3 and
Figure 4 ), with a larger effect of genotype in pubertal children when compared with prepubertal children (F=2.97, df=2, 1763, p=0.05). No main effects of puberty were found.
Discussion
This large study of the effect of COMT genotype on children’s cognition found little effect of genotype on girls but widespread effects in boys on measures of executive function and IQ, especially when pubertal development was considered.
The gender distinction has not previously been reported, but few previous studies have been large enough to detect such effects. However, there is evidence that COMT’s effects may be gender-specific. Dopamine levels in the frontal cortex of COMT knockout mice are affected only in male mice, and while male knockout mice show increased aggression, females show decreased emotional reactivity
(4) . In humans, COMT activity is lower in female subjects than in male subjects in dorsolateral prefrontal cortex tissue, and in lymphocytes, genotype affects enzyme activity in male subjects more than in female subjects
(33) .
It is not yet known whether human COMT expression differs in the brains of prepubescent boys and girls, but the cortical organizational changes that occur during adolescence do differ between the genders, with boys showing apparently greater age-related synaptic pruning and myelination than girls
(34) . These changes may conceivably be triggered by differences in the nature or level of gene expression. There are also significant gender differences in the incidence of neuropsychiatric disorders associated with COMT genotype such as schizophrenia and ADHD, both of which are more common in male subjects.
The peak onset of schizophrenia in male subjects is around late adolescence or early adulthood
(35), with cases before puberty being extremely rare. The cognitive deficit present in children before illness onset also worsens around the onset of puberty
(36) . Therefore, the biology of schizophrenia is probably related to those of puberty and brain development, and genes that interact with these processes may be crucial.
Puberty was measured here by parent rating of the child’s development according to Tanner stage. This approach was pragmatic given the large sample but remains imperfect when compared with expert examination of the children. However, these results appear to be within the range of normal development reported in samples from the United States
(37) and Italy
(38) .
The effect of genotype on cognition was increased in boys when pubertal stage was included in the model. One possibility is that boys at the same developmental stage are more homogenous, so subtle effects can be detected that tend to be masked when prepubertal and pubertal children are considered together. Our hypothesis suggested that puberty would interact with the gene-cognition relationship such that genotype would have a stronger effect post-puberty. Although the children were too young for us to investigate the effects of COMT post-puberty, we assessed its effects in children prior to, versus already in, puberty. We suggest that the increase in effect size in the puberty models, and the fact that with the exception of IQ and working memory the effects cannot be detected unless pubertal stage is included, suggests neurobiological development is an important factor.
The possible interaction between gene and puberty in verbal IQ supports our hypothesis regarding the increasing effect of COMT on cognition as the frontal lobes come online. We did not find a clear interaction between gene and puberty in the majority of tests studied. This may be due to the numbers involved: the effect of COMT genotype on verbal IQ was the most robust result in the group as a whole; it may be that weaker genotype-by-puberty interactions were present but not detectable given the small numbers of children in the pubertal group.
Modeled COMT effects explained around 1% of the variance in childhood cognitive performance compared with around 4% in adult studies
(6,
10) . This may reflect increased specialization in the adult brain, or developmental differences in the importance of COMT versus other enzymes in prefrontal cortex, such as the monoamine oxidases. On the other hand, the size of the present study was sufficient to detect small effects that may be missed by smaller studies with limited power. The ALSPAC cohort represents the largest study to date of the cognitive effects of COMT in patients, healthy adults, or children. Even with this large group size, effects would not have been considered significant if strict corrections for multiple comparisons (e.g., Bonferroni corrections) were adopted. We did not follow this approach because of the hypothesis-driven nature of our strategy, the restricted phenotype that we considered, the intercorrelation between cognitive outcomes, and the increased probability of type II errors if such corrections had been applied. We report all the statistical tests that we undertook, and we were reassured that all main effects were in the hypothesized direction, suggesting that type I errors did not account for the results. Nevertheless, independent replication will be essential, as it is for all genetic studies.
Demonstrating an effect of COMT on IQ scores is important for several reasons. Performances on diverse tests of cognitive function tend to correlate; this underlying covariance represents general cognitive ability, or ‘
g ’. Neuroimaging studies have demonstrated that in adults, ‘high-
g ’ tasks do not show diffuse recruitment from multiple brain systems but instead are associated with relatively selective recruitment of the lateral frontal cortex
(39) . It is therefore logical that COMT would affect general intelligence as well as executive tasks.
Intelligence, like risk for neuropsychiatric disorders, doubtless involves multiple genes of small effect. Research has consistently found
g to be strongly heritable
(40), but population-based studies have generally failed to establish genetic effects on cognitive development in children
(41,
42) . COMT may be the first gene that shows a plausible biological mechanism and has a relatively large effect across the normal range of IQ in children.
In schizophrenia, cognitive impairments present before the onset of psychosis reflect ongoing, aberrant neurodevelopmental processes that may worsen around the onset of puberty
(36) . The increased prevalence of the
Val allele in patients with schizophrenia
(7) may explain a proportion of the IQ decrement in children who will later develop schizophrenia. This is estimated to be around 5–10 points and is more pronounced in male subjects
(43) ; here we found a difference of 2–10 points in males attributable to COMT genotype.
It is possible that the effects attributed to the
Val 158/108 Met polymorphism are in fact due to some other factor. There is now substantial evidence that haplotypes spanning the COMT gene, including other single nucleotide polymorphisms (SNPs) as well as
Val 158/108 Met, are stronger predictors of risk for schizophrenia than that polymorphism alone
(44,
45) . These may reflect the importance of other sites that are in strong linkage disequilibrium with the
Val 158/108 Met SNP, or regulatory factors such as promoter regions
(45) .
The true complexity of COMT expression and function remains obscure but for the moment, we assume that
Val 158/108 Met genotype does affect catecholamine degradation in human prefrontal cortex. This is probably reasonable: Chen et al.
(33) studied the effects of
Val 158/108 Met and three other SNPs on mRNA levels, protein levels, and enzyme activity in human prefrontal cortex tissue and in lymphocytes and concluded that
Val is the predominant factor determining COMT activity in the prefrontal cortex. While other polymorphisms may still prove important,
Val 158/108 Met is the most well-characterized polymorphism, with a plausible biological mechanism. Moreover, the relatively restricted cognitive phenotype that we have demonstrated to be associated with this SNP has a strong relevance for the cognitive deficits prominent in schizophrenia.