Schizophrenia is a psychotic disorder that alters patients’ perception, thought processes, and behavior as evidenced by hallucinations, delusions, disorganized speech or behavior, social withdrawal, and varied cognitive deficits
(1) . Additionally, episodic memory and attention are significantly impaired in schizophrenia
(2) . Disturbed integration of activity across multiple brain regions or dysfunctional connectivity between frontotemporal brain regions is a central feature of schizophrenia
(3,
4) . Symptoms of schizophrenia have been attributed to a failure of functional integration or aberrant connectivity among regions or systems of the brain
(4) .
The “default mode” network has been identified as a resting state of brain function; this network has repeatedly exhibited significant functional connectivity across a wide variety of tasks and during a conscious inactive state
(5 –
11) . Brain regions identified as parts of the default mode network include the posterior cingulate and medial, prefrontal, parahippocampal, and inferior parietal cortices, among others
(10) . Although the exact roles of the default mode network are unknown, it has been implicated in attending to external and internal stimuli
(5,
6,
12), as well as self-referential and reflective activity
(5,
7,
11) that specifically includes episodic memory retrieval, inner speech, mental images, emotions, and planning of future events
(9,
13) . McKiernan et al.
(10) found that task-induced deactivation of the default mode network increased as task difficulty increased in a subsequently administered cognitive probe task. They hypothesized that this was due to a reallocation of processing resources from the default mode network to areas used in task performance. This is consistent with a hypothesis that the default mode network is involved in ongoing information processing; as task demand increases, fewer attentional resources can be devoted to this default mode network
(8) .
Because the mental processes involved in the default mode are relevant to schizophrenia, it was hypothesized that the default mode network would be abnormal in these patients. Inasmuch as the default mode network is involved in many aspects of brain function, its healthy functional connectivity is imperative to normal mental function; impaired connectivity or activation, as seen in other brain networks in schizophrenia, might influence positive and negative symptoms of the disorder.
Our goal was to test the hypothesis that the default mode might show abnormal spatial and/or temporal patterns of activity in schizophrenia. Independent component analysis, a technique that maximizes the independence between the output components, was used to analyze the data
(14,
15) . The independent component analysis algorithm attempts to determine a set of nonsystematically overlapping (spatially independent) brain networks, each with associated time courses. The default network is readily (and unambiguously) identified by using independent component analysis
(8) . In its application to functional magnetic resonance imaging (fMRI) data, independent component analysis separates the fMRI data into a set of spatially distinct networks and their temporal signatures and is useful for revealing functionally related, although not necessarily task-related, cortical areas
(16) . We used independent component analysis to calculate spatially independent, temporally synchronous regions; the default mode component for each subject was selected with a spatial template to identify the component of interest. We extracted the default brain modes from fMRI data collected during the performance of an auditory oddball task. We employed the auditory oddball task as a type of control (as opposed to an uncontrolled resting state scan) and also to stimulate the brain with a relatively simple task that elicits robust brain function differences between comparison subjects and patients
(17) . Patients with schizophrenia perform as accurately as healthy comparison subjects, although they have slightly longer response times
(14,
17) . The oddball task is also well suited to show whether the fMRI signal in these networks is modulated by behavioral performance, something that is not possible when analyzing resting-state scans.
Discussion
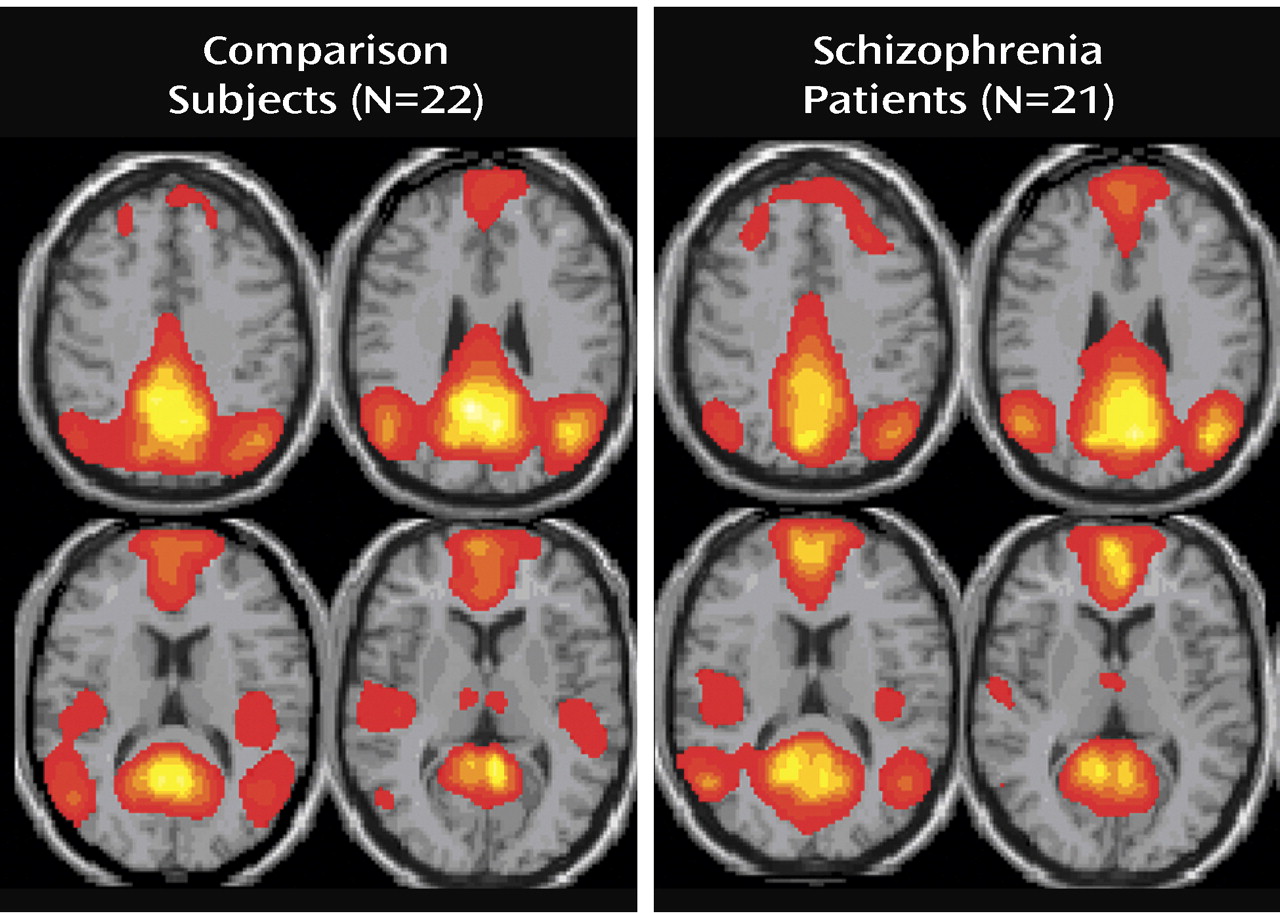
This study demonstrates that schizophrenia is associated with both temporal frequency alterations and disruption of local spatial patterns in the default mode network. The default mode was identified as the only component that correlated significantly and most negatively with both the WFU Pick atlas template and the auditory oddball task in both patients and comparison subjects
(7,
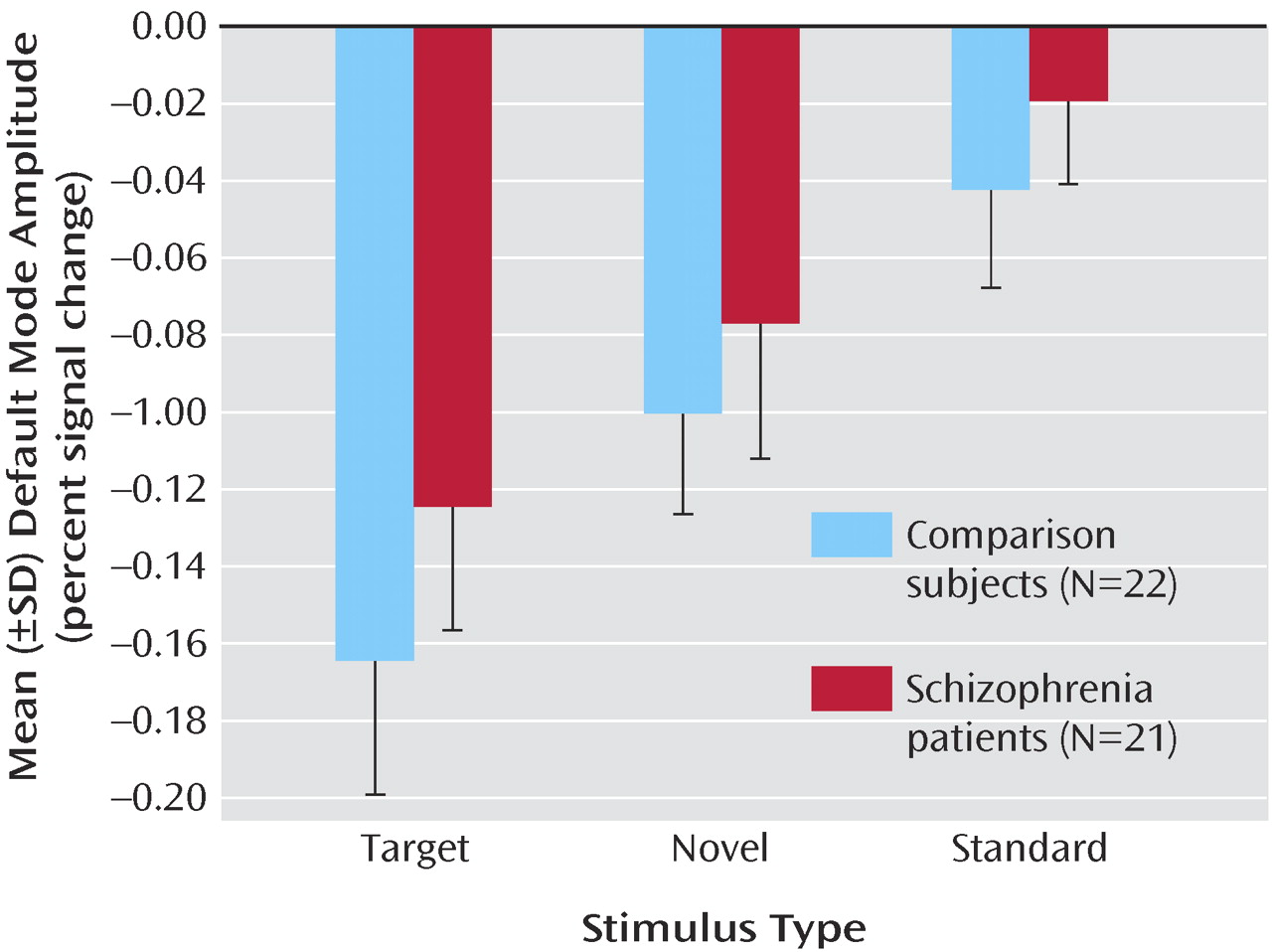
8) . Although the default mode spatial maps looked similar for patients and comparison subjects, there were several interesting differences. Spatially, both patients and comparison subjects strongly correlated with the template; however, comparison subjects showed higher correlation, perhaps indicating more variability in the patient maps. Target stimuli produced the largest decrease in default mode activity, followed by novel then standard stimuli. This is consistent with previous research that suggests that increased task difficulty/attentional load leads to increased default mode deactivation, possibly as cognitive resources are reallocated to task-related regions
(10) . Patients tend to exhibit smaller default mode changes overall than do healthy comparison subjects; however, this finding was not significant. This tendency may be related to the attentional deficits in schizophrenia and to symptoms such as distractibility and focus on irrelevant internal and external stimuli. This raises the question as to whether patients would show less deactivation in relation to healthy comparison subjects during a more demanding task.
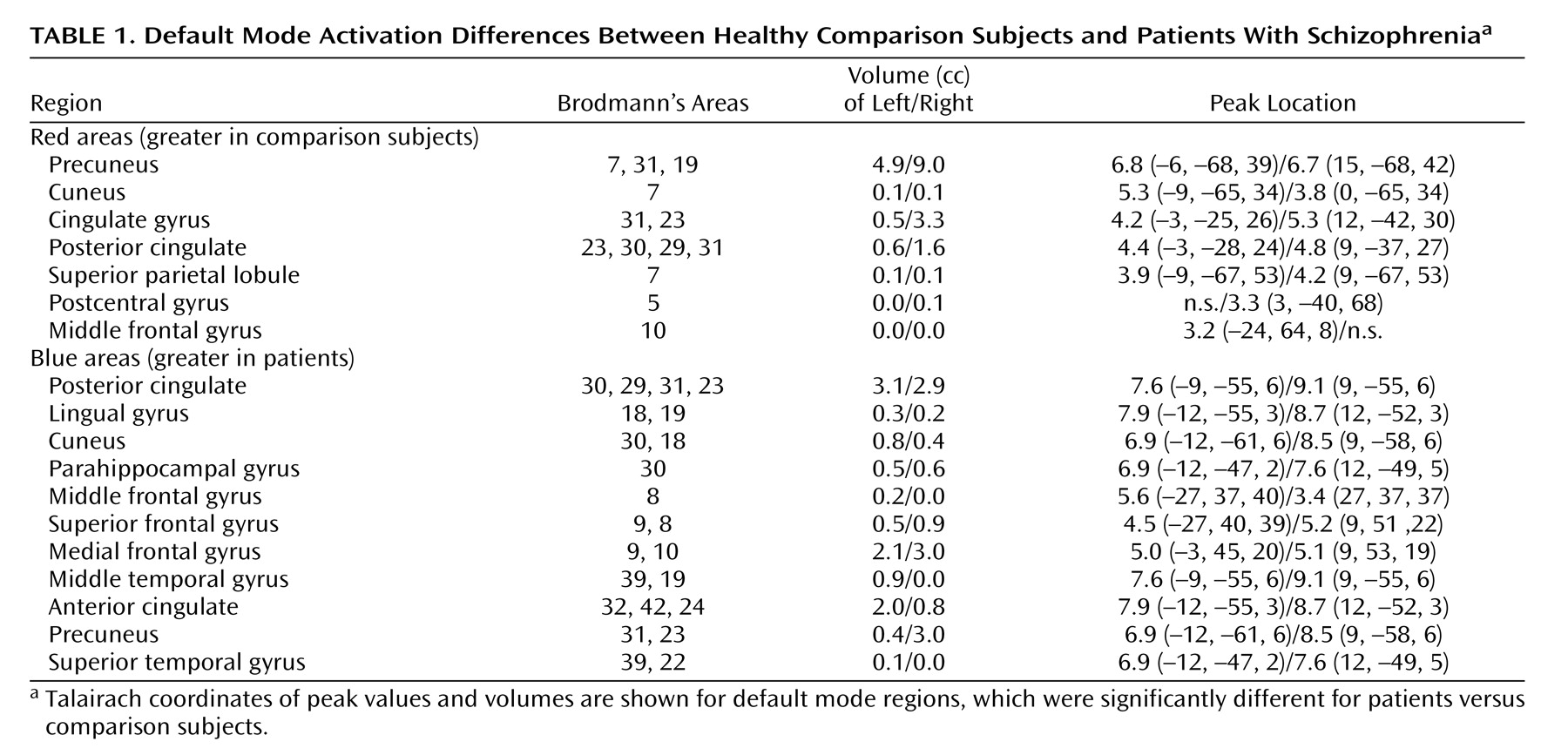
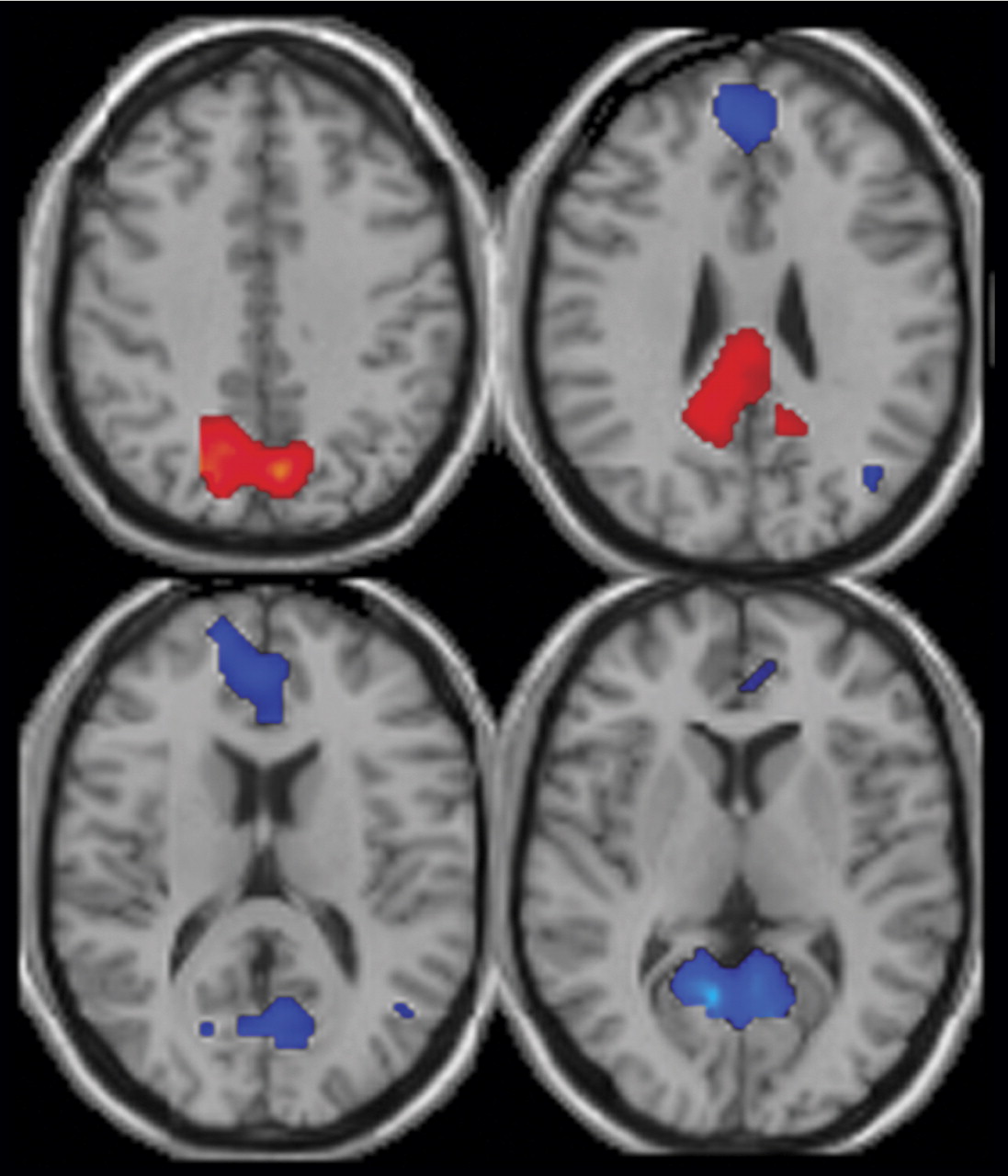
Three major differences in the spatial pattern of default mode activity were observed between patients and comparison subjects. First, patients showed greater deactivation in areas of the frontal gyrus involved in the default mode. Second, patients showed decreased activation in the anterior cingulate relative to comparison subjects. Third, a larger region of the parahippocampal gyrus was included in the default mode of patients versus comparison subjects. The wide variety of regions exhibiting bilateral differences in the default mode network of patients suggests that the strong connectivity of this network
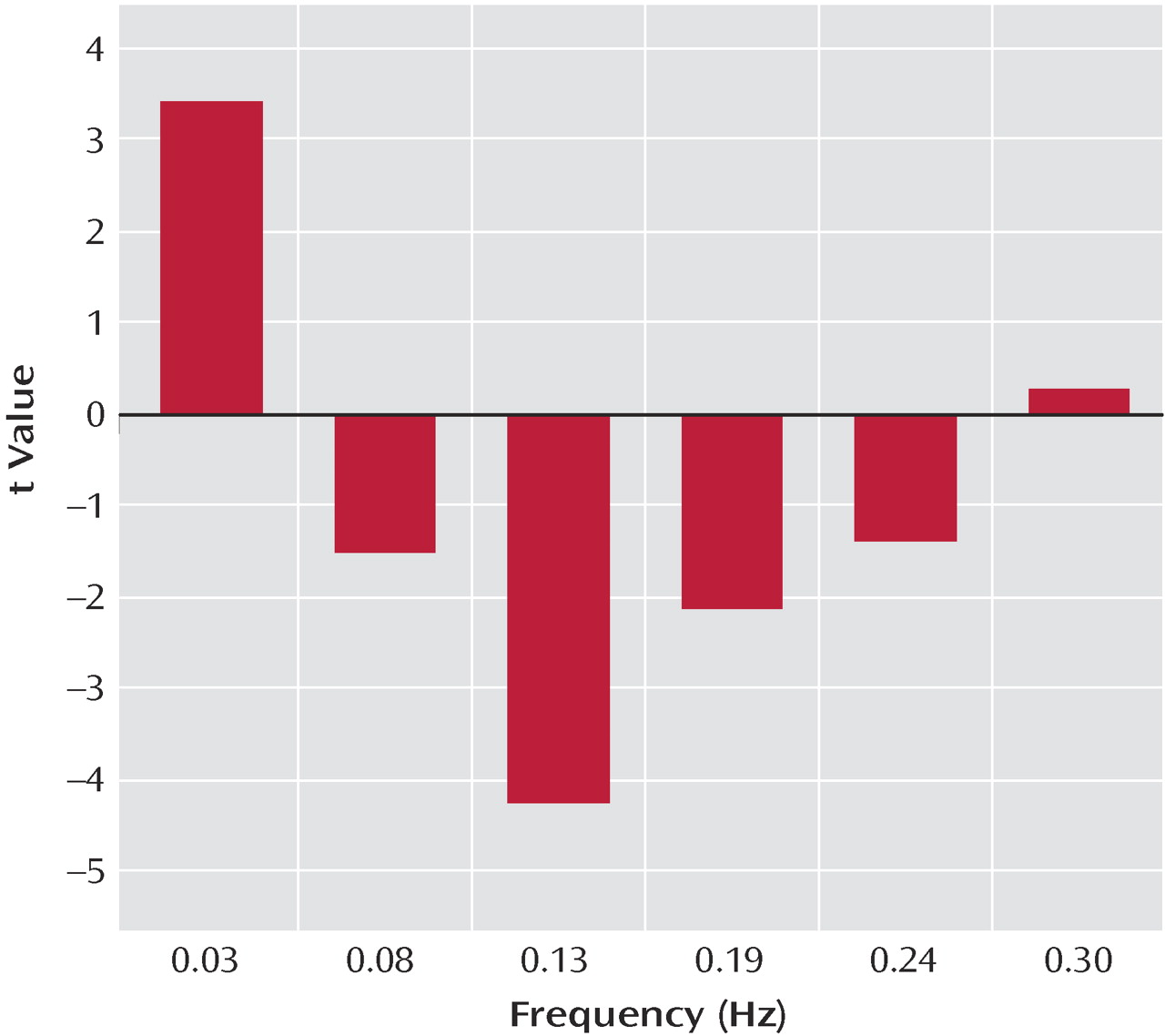
(7) is altered in schizophrenia patients. Additionally, healthy comparison subjects exhibited low-frequency oscillations (0.03 Hz), whereas patients exhibited significantly higher-frequency oscillations in the 0.08–0.24 Hz range. This result may indicate less temporal synchronicity between the brain regions involved in the default mode network of patients or may even indicate impaired communication between the default mode network and other brain regions in schizophrenia.
The patients with schizophrenia showed greater deactivation in the middle, medial, and superior frontal gyrus bilaterally in the default mode component. Previous studies have demonstrated similar “hypofrontality” in patients
(39) . Cognitive disorganization has been suggested as one cause of decreased activation in the prefrontal cortex
(20), and dorsolateral prefrontal cortex dysfunction has been related to formal thought disorder and attentional deficits of schizophrenia
(40) . Abnormal integration of frontal-temporal function, underpinned by a failure of normal cingulate cortical modulation, has also been demonstrated in patients
(3) .
In patients with schizophrenia, the anterior cingulate showed a reduced volume of activation, more on the left, in relation to comparison subjects. In addition, patients had greater deactivation bilaterally during the task. Abnormal fMRI activation of the anterior cingulate is widely reported in schizophrenia and has been correlated with working memory deficits
(3) . The anterior cingulate is believed to play a role in modulating basic subconscious and higher cortical processing in the default mode during rest
(7) and monitoring task progress and implementing task strategy
(41) . A failure to monitor internally generated actions has been implicated in hallucinations and delusions in schizophrenia; the activation of the anterior cingulate in both a resting state and during a task implicates it as a modulator of default mode functioning. Its inability to function correctly to modulate internal thoughts and those related to the task may play a role in the positive symptoms of schizophrenia.
Significant differences in hippocampal morphology, volume, neuron size, and connectivity are reported in schizophrenia
(18) . In this study, the parahippocampal gyrus was found to have decreased deactivation bilaterally. Parahippocampal neuropathology may play a significant role in the episodic memory problems and related cognitive deficits in schizophrenia and may also be associated with negative symptoms
(18,
19,
22,
42) . The hippocampus is functionally connected to brain regions involved in working memory
(42), such as the dorsolateral prefrontal cortex
(19) . The decreased deactivation of the frontal cortex and parahippocampal gyrus found in this study may be caused by an altered functional connectivity between these two regions, as demonstrated in previous studies
(18,
19) . Positive symptoms have previously been associated with increased hippocampal activation at rest, as was found in this study
(22) .
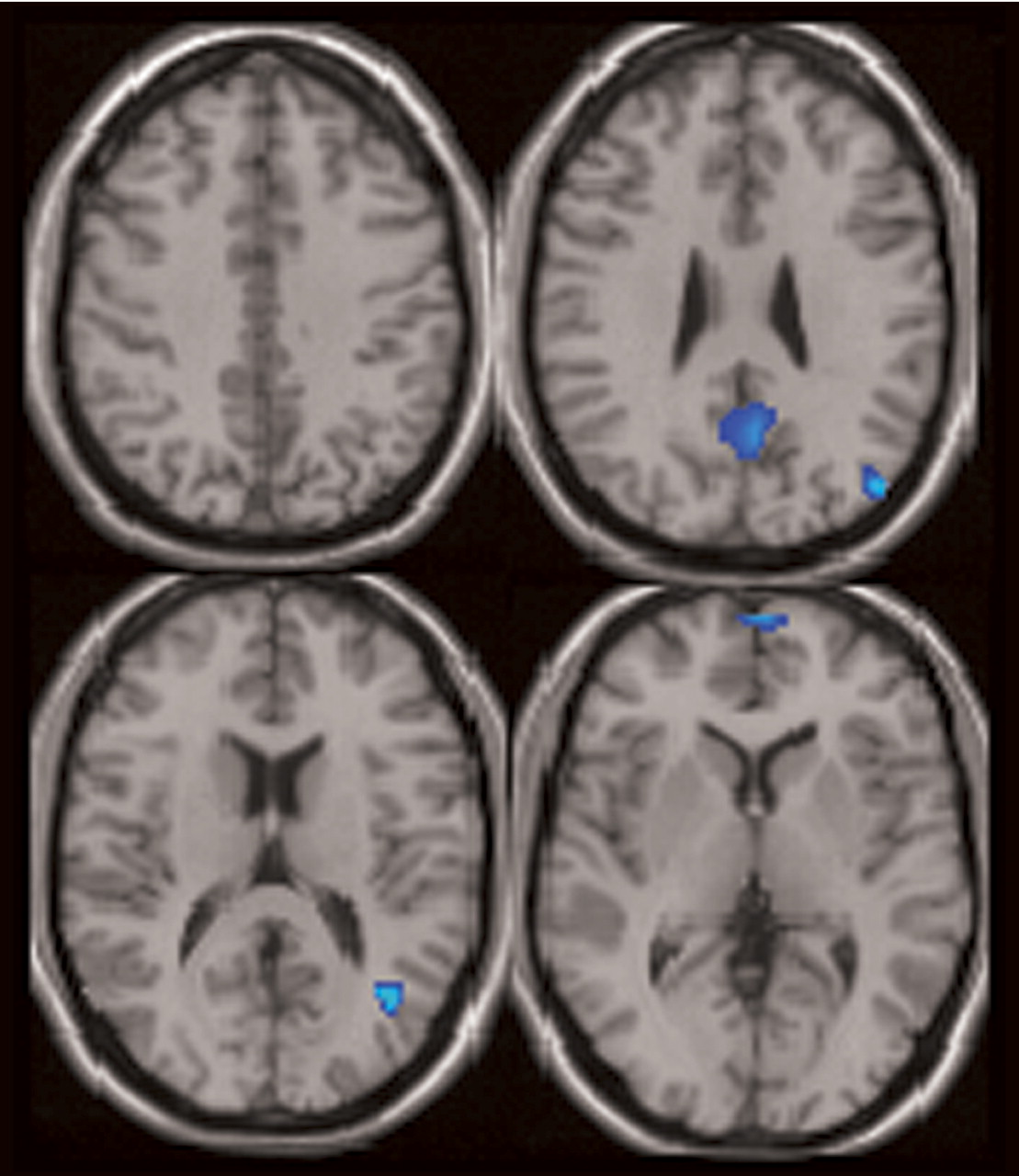
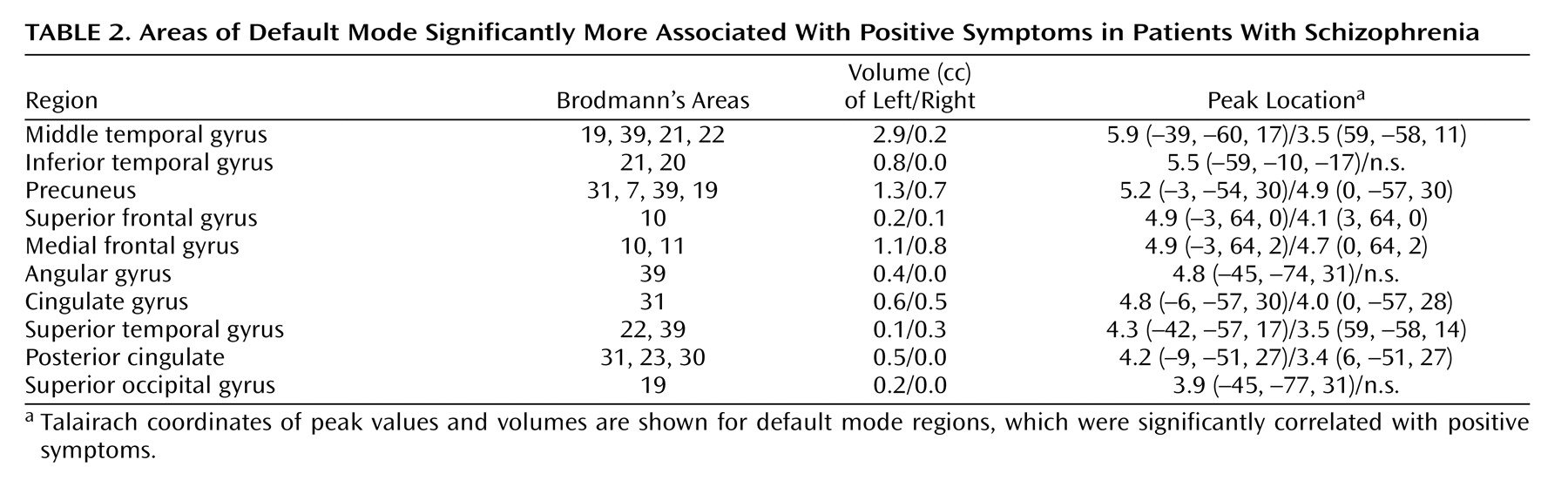
The medial frontal gyrus, left middle and inferior temporal gyri, precuneus, cingulate gyrus, and left posterior cingulate all correlated with positive symptom severity (
Table 2 ). Positive symptoms have previously been associated with abnormal activation of the prefrontal cortex
(20), superior temporal gyrus, and anterior and posterior cingulate
(21,
40) .
The default mode consistently shows low-frequency blood-oxygen-level-dependent (BOLD) signal fluctuations (0.012 Hz to 0.1 Hz) that contribute to functional connectivity in healthy comparison subjects
(8,
11) . These low-frequency fluctuations represent temporal synchronicity among functionally related regions of the brain. In this study, patients and comparison subjects exhibited low-frequency oscillations in BOLD signals at similar frequencies. However, comparison subjects had significantly more power in this low-frequency range and patients exhibited significantly higher power at higher frequencies (
Figure 4 ). The high-frequency oscillation observed in this study is comparable to that found in a temporal lobe network
(14) and in a small study of five patients with schizophrenia and matched healthy comparison subjects. An unpublished study by Calhoun et al. found that the time courses for the temporal lobe network in patients showed a tendency toward more power at higher frequencies than those of normal comparison subjects. The greater fluctuations BOLD signals observed in the default mode time course components for patients in this study may reflect a lower degree of interconnection between the regions in the default mode and other brain regions (e.g., cognitive dysmetria)
(43) .
Altered functional connectivity in schizophrenia has been demonstrated in many areas that evidenced altered activation in this study, including the parahippocampal and prefrontal cortices
(18,
19) . Furthermore, abnormal modulation of brain regions has also been implicated in the symptoms of the disorder
(44) . The anterior cingulate has been shown to play a role as a modulator of internal speech and attending to the outside environment and also demonstrated altered activation in this study
(41) . The default mode, or regions of it, may be involved in overactivity of brain regions that interfere with normal thought and functioning, thus disrupting the “internal monologue” and playing a role in the genesis of delusional thoughts and hallucinations. Previous research suggests that auditory hallucinations, experienced by patients in this study, may be due to a failure to properly interpret inner speech
(45) .
An advantage of our use of individual independent component analysis in this study is that it allowed for examination of functional connectivity without specifying regions of interest beforehand. In this study, independent component analysis is consistent with other methods of data analysis for the identification of the default mode network. Independent component analysis also revealed aspects of brain activity in schizophrenia that may not be apparent through other forms of analysis. These include the differences in temporal characteristics of default mode activity and the individual differences among subjects, which will inform future research and may lead to further understanding of the mechanisms underlying schizophrenia. Another advantage of this study is the large group size. The degree to which the auditory oddball task affected the default mode is unknown because there is no previous research using the auditory oddball task to examine the default mode, to our knowledge.
This research provides strong evidence for a significantly different default mode network in patients with schizophrenia. Regions previously identified to be abnormal in schizophrenia evidence abnormalities in the default mode. In addition, abnormal cingulate modulation of the default mode may play a role in the auditory hallucinations, delusional thoughts, and attentional deficits that are hallmarks of schizophrenia.