The pathophysiology of schizophrenia remains unknown, although functional neuroimaging studies have consistently demonstrated the involvement of frontal and temporal cortical regions
(1 –
3) . Cognitive models propose that the symptoms of the disorder reflect a disruption of frontal-temporal functional connectivity
(4), and there is also evidence of disrupted frontal-temporal and frontal-parietal connectivity from functional neuroimaging
(5 –
6), volumetric imaging
(7), and electrophysiological investigations
(8) . Nevertheless, the extent to which these findings reflect differences in the underlying anatomical cortical-cortical connectivity is unclear.
Diffusion tensor imaging data are acquired through the modification of a conventional magnetic resonance imaging (MRI) sequence to permit quantification of the diffusion characteristics of water molecules in vivo
(9) . Within cerebral white matter, water molecules diffuse more freely along myelinated tracts than across them
(10) . Such directional dependence of diffusivity is termed “anisotropy,” and any reduction in white matter anisotropy indicates a reduction in the degree of tissue order on the voxel scale. Such structural changes may reflect changes to the underlying white matter tracts, and diffusion tensor imaging can thus be used to examine anatomical connectivity in vivo. However, recent applications of diffusion tensor imaging to schizophrenia have yielded inconsistent results
(11 –
22) . Although several studies have reported decreased fractional anisotropy in schizophrenia, there is much less consistency in the topographic location of the white matter changes. This may be related to variations in methodology, both during acquisition (e.g., the extent of brain coverage and the resolution of the images) and during analysis (the relative group size, the selection of the anisotropy measure to be assessed, and whole brain versus region-of-interest approaches). The corpus callosum has been the most frequently reported site of differences
(11 –
13,
15,
21), although this may be related to the fact that it is the region most frequently examined.
In the present study, we used an optimized diffusion tensor imaging acquisition sequence, permitting whole brain coverage with excellent image resolution, within a time tolerable for patients. We then employed an analysis based on automated voxel-based comparisons with conservative-permutation-based statistics to examine changes in fractional anisotropy (one of a number of quantitative measures of anisotropy that can be extracted from the diffusion tensor) in a relatively large group of patients with schizophrenia and matched healthy volunteers. Our first hypothesis, based on the evidence of functional dysconnectivity, was that patients would show reduced fractional anisotropy in the major white matter tracts connecting the frontal cortex with the ipsilateral temporal and parietal cortices
(4 –
8) . These tracts include the superior longitudinal fasciculus, which links Wernicke’s and Broca’s areas, and the uncinate fasciculus, which connects the orbitofrontal and temporal polar cortex. A second prediction, based on data from previous diffusion tensor imaging studies
(11 –
13,
15,
21), was that there would also be reduced fractional anisotropy in the genu of the corpus callosum. Finally, in view of recent diffusion tensor imaging data linking auditory hallucinations in schizophrenia with changes in increased fractional anisotropy in the arcuate fasciculus and anterior corpus callosum
(15), we tested the hypothesis that fractional anisotropy in these regions would correlate with propensity to hallucinations.
Discussion
Despite being associated with marked symptoms and cognitive dysfunction, schizophrenia is associated with relatively subtle neuropathological changes in cortical gray matter
(33) . The absence of marked gray matter changes, as well as recent evidence from functional neuroimaging and electrophysiological studies
(2,
4,
8), is consistent with cognitive models that propose that schizophrenia also involves a disturbance in the connections between gray matter areas. In the present study, we used diffusion tensor imaging in conjunction with a voxel-based analytical approach to examine white matter architecture in schizophrenia. Because it is automated, it reduces the risk of errors due to user intervention and avoids the inherent difficulty in placing regions of interest. We applied this to a group of patients that is larger than has previously been studied with diffusion tensor imaging.
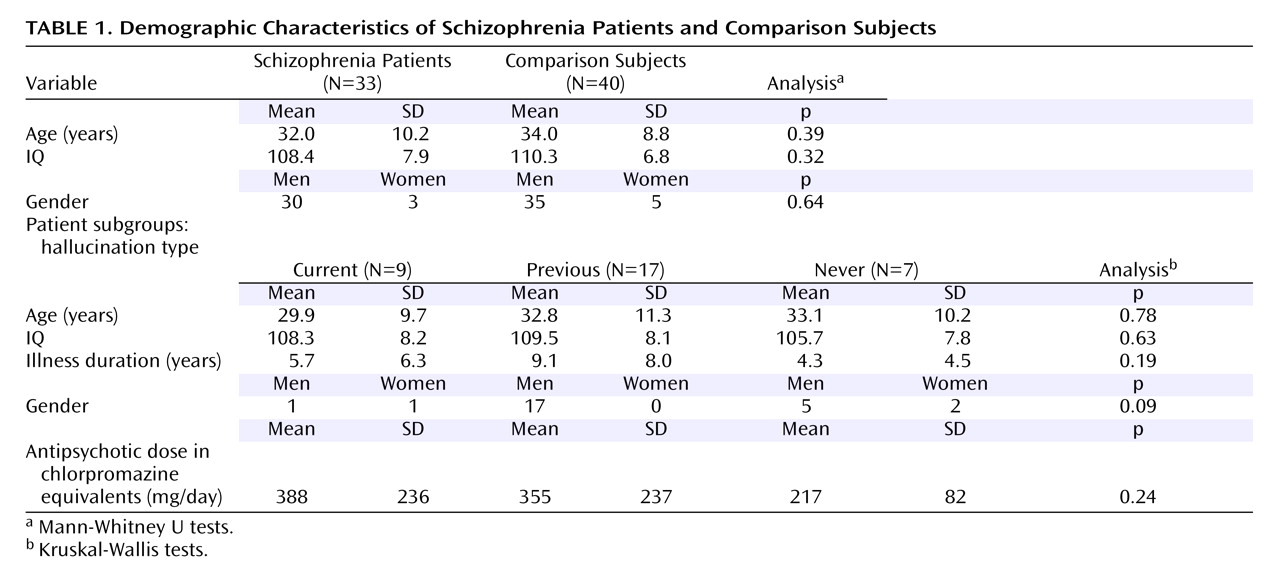
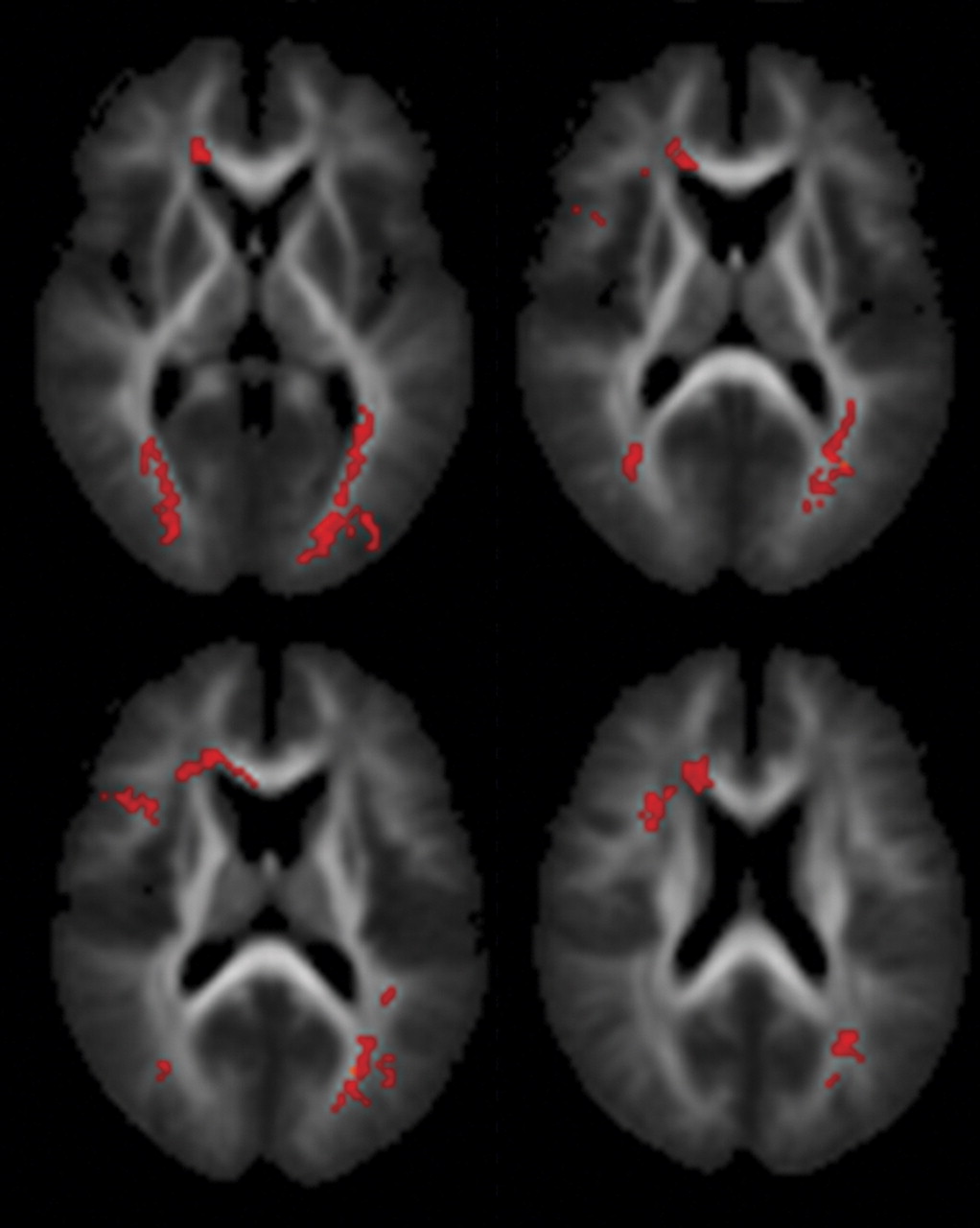
As predicted, we found reduced fractional anisotropy in the superior longitudinal fasciculi bilaterally, consistent with previous diffusion tensor imaging data
(15) . The superior longitudinal fasciculus is a major association tract connecting large parts of the frontal association cortices with parietal and temporal association areas
(30) . It forms the main connection between Wernicke’s and Broca’s areas, which is of particular interest because abnormal language processing (manifest clinically as formal thought disorder and auditory verbal hallucinations) is a key feature of schizophrenia. Contrary to our hypothesis, we did not find reduced fractional anisotropy in the uncinate fasciculus, the other major tract connecting frontal and (anterior) temporal regions, or the cingulum, both of which have shown differences in previous studies
(17,
18,
20 –
21) . This may have been secondary to differences either in patient samples or in the methods used during image acquisition and subsequent analysis, particularly the use of voxel-based methods that allow whole brain testing but may be less powerful than discrete region-of-interest approaches.
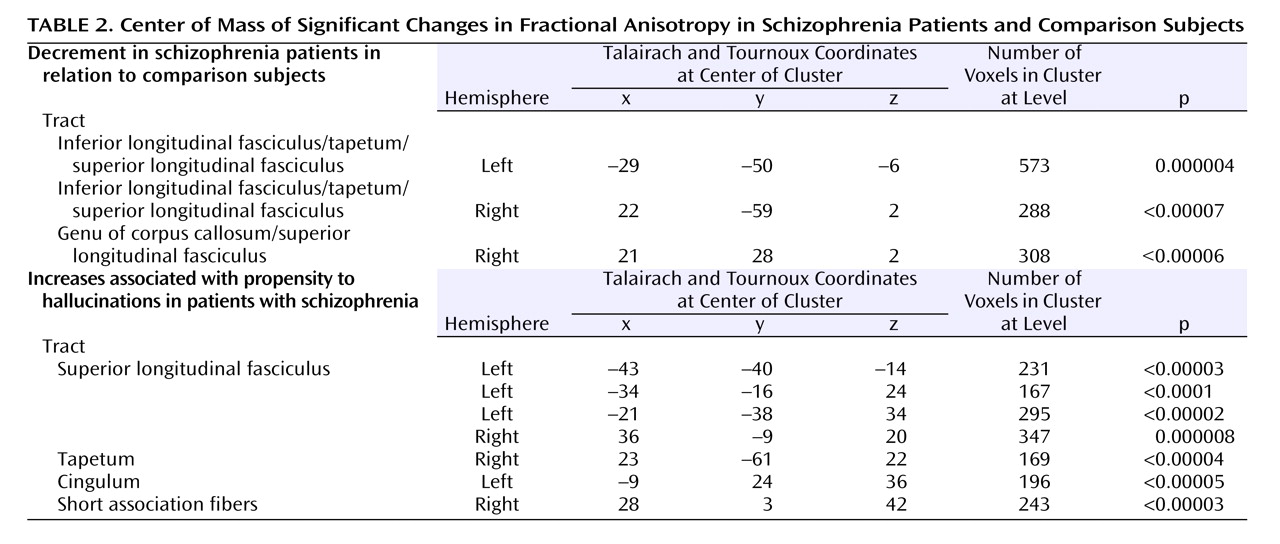
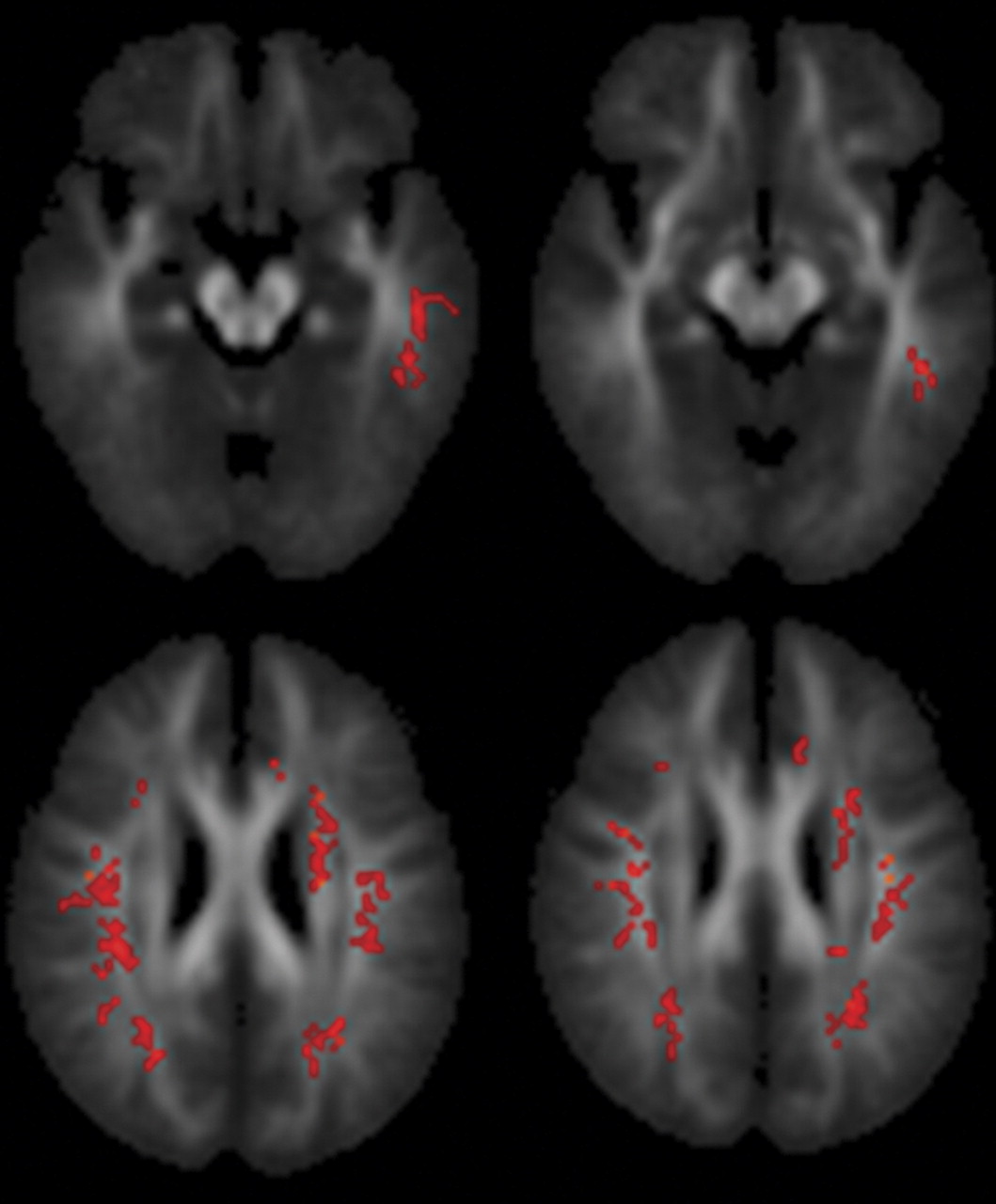
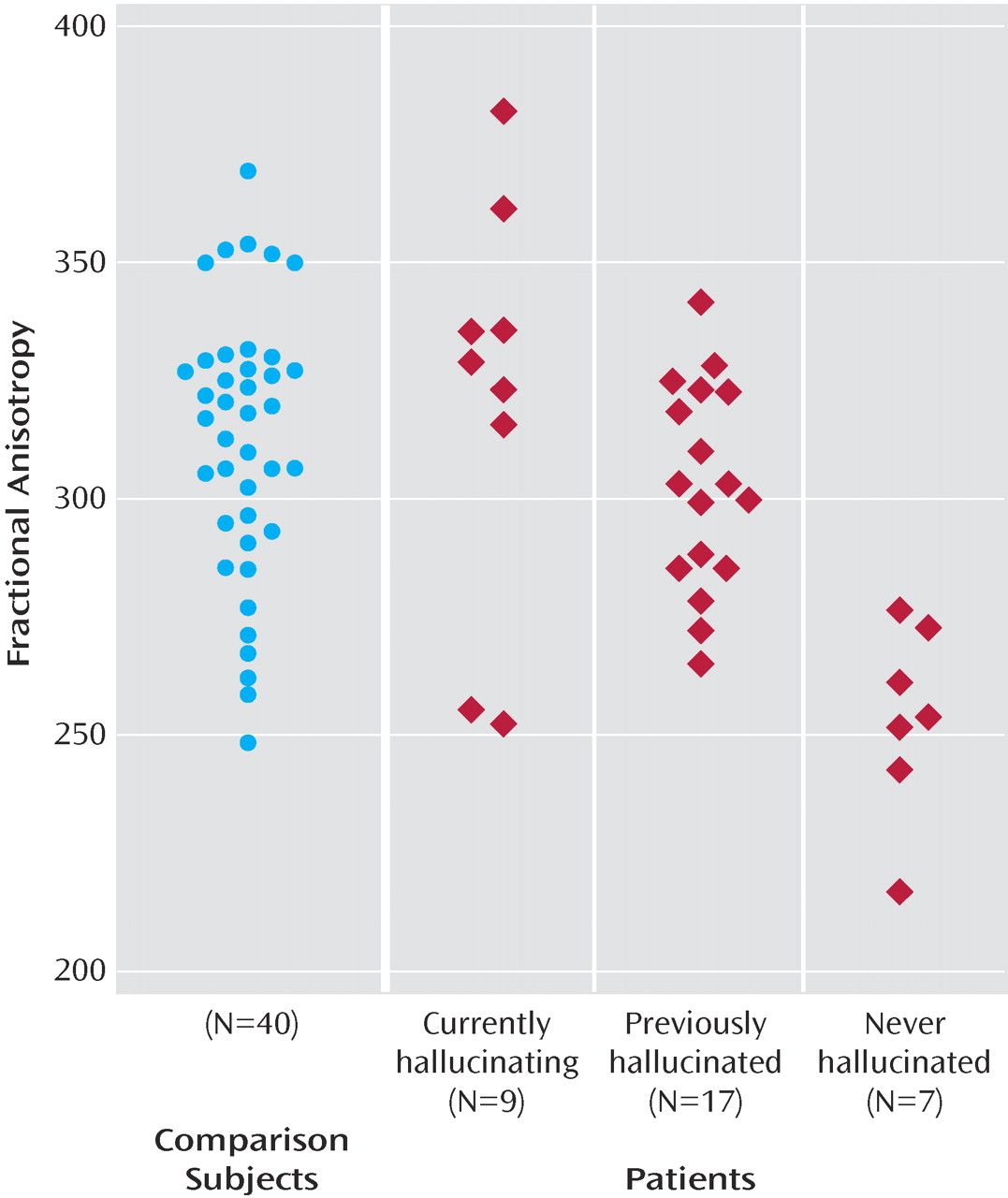
Within the patient group, proneness to auditory hallucinations was associated with increased fractional anisotropy within an inferior temporal region of the left superior longitudinal fasciculus and a more superior region bilaterally. However, even in the patients who were most prone to hallucinations, the fractional anisotropy in this region was still lower than in healthy comparison subjects. These findings are broadly consistent with those from a recent study that reported relatively increased fractional anisotropy in a similar part of the superior longitudinal fasciculus (the coordinates of their region of interest were at –42, –38, 4, compared with –43, –40, –14 in this study) with vulnerability to hallucinations, although they reported fractional anisotropy differences that were also significantly greater than their healthy comparison group
(15) . Although fractional anisotropy changes can be caused by a variety of factors
(28), a relative increase in fractional anisotropy within these frontotemporal tracts, especially within these more lateral aspects, could reflect greater connectivity between the lateral frontal and temporal cortex, which might perturb normal communication between areas involved in the generation and monitoring of inner speech
(6,
34) and contribute to the abnormal coactivation of these regions in functional imaging studies of patients with hallucinations
(1 –
2) . However, it is also possible that changes in connectivity may occur as a secondary phenomenon to experiencing auditory hallucinations, with the connections enhanced with the frequency of the hallucinatory experience. This issue could be examined in longitudinal studies of patients with hallucinations, ideally with groups of matched nonhallucinating patients.
The most consistent abnormality in previous diffusion tensor imaging studies of schizophrenia has been reduced fractional anisotropy in the corpus callosum
(11 –
13,
15,
18,
21), and we also found reduced fractional anisotropy in this region, within its anterior portion (the genu). Previous diffusion tensor imaging findings in the corpus callosum have mainly been localized to either its posterior portion
(11,
18) or to both its anterior and posterior portions. The present findings are compatible with previous reports of decreased volume of the genu in schizophrenia
(35) and evidence of functional dysconnectivity between the frontal cortex and contralateral temporal and parietal cortex
(4 –
6,
34) .
It was difficult to precisely localize the decreased white matter integrity within the region close to the junction of the tapetum and the inferior longitudinal fasciculus. However, based on a diffusion tensor imaging study that demarcated the inferior longitudinal fasciculus with tractography
(36), the main area of decreased anisotropy in the present study appears to lie within the tapetum. This is part of the splenium of the corpus callosum connecting homologous regions of the temporal lobes.
Most of the patients were taking psychotropic medication at the time of scanning. Antipsychotic medication has been suggested to influence fractional anisotropy, with one laboratory reporting increased fractional anisotropy with increased antipsychotic dose
(19,
20) . Therefore, we cannot exclude the possibility that differences in fractional anisotropy between patients and comparison subjects were related to medication; however, similar differences have been described in first-episode patients who had received only a few days of antipsychotics and in individuals at high risk of psychosis who were medication naive
(37) . Similarly, the differences between subgroups of hallucinators were unlikely to be secondary to antipsychotic treatment because neither the current dosage nor the cumulative dosage—as indexed by illness duration—differed significantly between these subgroups (
Table 1 ). Although age has an effect on anisotropy, this was included as a covariate within the analysis. In view of evidence that there are progressive volumetric changes in schizophrenia
(38), duration of illness may also affect fractional anisotropy. However, in the present study, length of illness was highly correlated with age (Pearson’s r>0.84), so covarying for age is likely to also have eliminated any effects of illness duration. Because of the limited resolution of the diffusion tensor imaging data (2.5-mm voxels), spatial localization errors may be introduced by the registration of data into standard space, and specific tracts were identified through cross-referencing to anatomical atlases. As a result, the localization of the findings to specific tracts is indicative rather than definitive. Diffusion tensor imaging data are exquisitely sensitive to movement artifacts, and it is difficult to exclude any contribution because of systematic differences between groups. An experienced image analyst assessed all the scans while blind to diagnosis and rejected those with movement artifacts. As expected, we had to reject a larger number of patients than comparison scans (15% versus 7%).
We thresholded the registered fractional anisotropy images to remove gray matter and CSF voxels and thereby minimize edge effects. Although the same threshold was applied to all images, other operator-independent procedures, such as segmenting the b=0 images or a coregistered T
1 -weighting, may have advantages in terms of identifying white matter. We used a combined affine and low-dimensional nonlinear registration to map images into standard space. Most previous voxel-based studies of diffusion tensor imaging measures have used either an affine
(14) or a similar combination of affine and low-dimensional nonlinear registration
(11) . Such registration algorithms do not produce exact spatial homology across individuals, and we used spatial smoothing to minimize any error. The use of smoothing inherently sensitizes the analysis to differences with a similar spatial extent to the smoothing kernel used; with no a priori hypothesis as to what size of change to expect, we chose to use only a very small degree of smoothing. This increases the risk of detecting changes that are due to misregistration rather than pathology, so we have replicated the findings in the anterior corpus callosum with a region-of-interest method
(39), indicating that at least in this region, the differences in fractional anisotropy were not an artifact of using a voxel-based approach.
In summary, the present study provides evidence of anatomical changes in the tracts connecting the frontal cortex with the temporal and parietal cortices and with the contralateral frontal and temporal lobes in patients with schizophrenia. These changes could underlie the functional dysconnectivity between these regions that has been previously reported in schizophrenia. They may also contribute to the symptoms and cognitive deficits associated with the disorder by interfering with normal mechanisms of motor and cognitive control that rely on rapid communication between distributed cortical regions
(4,
40) .