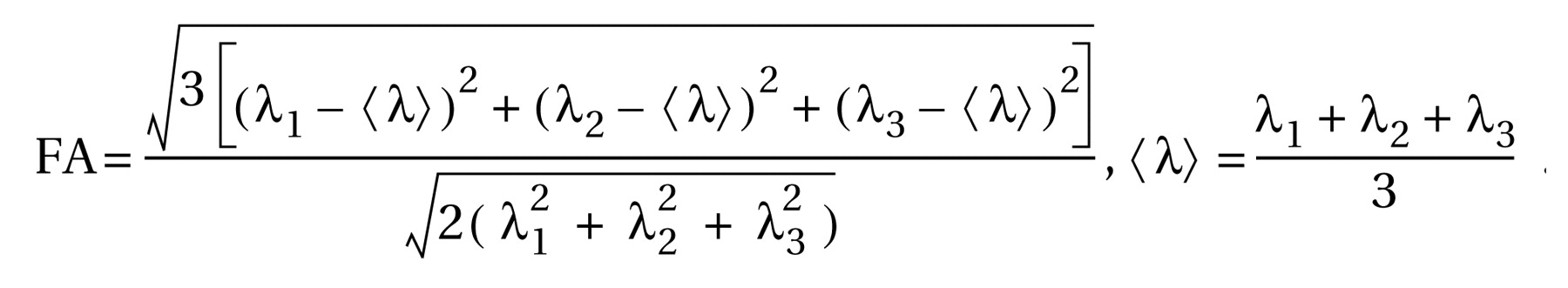Abnormalities in frontal-subcortical circuits may play an important role in the pathophysiology of major depressive disorder
(1 –
4) . Magnetic resonance imaging (MRI) studies of depression have demonstrated reduced gray matter volumes in the prefrontal cortex
(5 –
7), including the middle frontal gyrus
(8), anterior cingulate, gyrus rectus, and orbitofrontal cortex
(9) . Also, deep white matter hyperintensities
(5,
10,
11), especially at the level of the dorsolateral prefrontal cortex, have been reported in elderly depressed patients
(12) . Prefrontal abnormalities in both gray and white matter are most strongly associated with cognitive and emotional dysfunction in depression
(2,
13) .
Diffusion tensor imaging, a newly developed MRI technique, can provide information about white matter microstructural integrity in vivo by measuring the magnitude and direction of water diffusion. Recently, diffusion tensor imaging studies using region-of-interest methods in elderly depressed patients found microstructural white matter abnormalities in frontal and temporal lobes
(14 –
17), although in one of these studies
(14) the influence of antidepressant medications could not be excluded. Despite growing evidence for prefrontal cortical abnormalities in young as well as in elderly depressed patients
(18), there are no published diffusion tensor imaging studies examining the integrity of whole-brain white matter in first-episode, treatment-naive young adult patients with major depressive disorder. We designed the pilot study reported here to further understand the neuropathology of major depressive disorder by using voxel-based analysis (19–21), a method that can assess comprehensive global brain structure changes without the restrictions imposed by the prior selection of regions of interest and thus potentially identify unsuspected anatomic abnormalities in the brain
(22) .
Method
Subjects
Fourteen right-handed outpatients with major depressive disorder (12 of them female) age 20–41 years (mean=28.9, SD=8.0) were recruited at Second Xiangya Hospital of Central South University in Changsha, Hunan, China. They were interviewed with the Structured Clinical Interview for DSM-IV. All patients were experiencing their first episode of major depressive disorder and were treatment naive. The mean age at illness onset was 28.1 years (SD=7.8) and the mean illness duration was 10.3 months (range=3 to 24, SD=8.3). Scores on the Beck Depression Inventory (BDI) at the time of the study ranged from 27 to 48 (mean=37.5, SD=6.9). Fourteen matched right-handed healthy comparison subjects (12 of them female) age 19–41 years (mean=27.1, SD=6.7) were also recruited. The two groups had similar years of education (patient group, mean=11.4 years [SD=3.5]; comparison group, mean=12.0 years [SD=2.8]; p>0.05).
Exclusion criteria for both patients and comparison subjects were any history of loss of consciousness, meeting DSM-IV criteria for substance abuse within the 6 months prior to the scanning, mental retardation, or a current serious medical or neurological illness. An additional exclusion criterion for the patient group was any lifetime psychiatric disorder other than major depressive disorder. Additional exclusion criteria for comparison subjects were any history of psychiatric illness or a family history of major psychiatric or neurological illness in first-degree relatives. All subjects were given information about the procedures and gave written informed consent via forms approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Image Acquisition and Processing
Diffusion tensor imaging was performed on a 1.5-T GE scanner (Twinspeed, Milwaukee). A birdcage head coil was used, along with restraining foam pads to minimize head motion. Single-shot echo planar diffusion-weighted imaging with alignment of the anterior commissure-posterior commissure plane was done, using the following parameters: repetition time=12,000 msec; echo time=105 msec; acquisition matrix=128×128; field of view=24×24 cm; number of excitations=5; slice thickness=4 mm, no gap, 30 contiguous axial slices. The diffusion sensitizing gradients were applied along 13 noncollinear directions (b=1000 s/mm 2 ), together with an acquisition without diffusion weighting (b=0).
The diffusion tensor matrix was calculated according to the equation in Basser et al.’s study
(23) . Three pairs of eigenvalues (λ
1, λ
2, λ
3 ) and eigenvectors can be obtained by diagonalization of the tensor matrix. The principal direction at each point was given by the eigenvector that corresponds to the largest eigenvalue, and a fractional anisotropy (FA) value
(24) was calculated according to the following formula:
For each subject, statistical parametric mapping (SPM2, Wellcome Department of Cognitive Neurology, London) was first used to normalize the b=0 image to the standard Montreal Neurological Institute (MNI) space by nonlinear registration, and then the transformation matrix was applied to the FA map in order to normalize the map to the standard MNI space. All images were resampled with a final voxel size of 2×2×2 mm 3, and a 6-mm full-width half-maximum Gaussian kernel was used with the FA map to decrease spatial noise and smooth the data. No correction for eddy current distortions was applied.
Statistical Analysis
Two-sample t tests were performed in a voxel-by-voxel manner. A p value (two-tailed) lower than 0.001 (uncorrected) in voxel difference and a cluster size greater than 50 voxels were considered to be statistically significant. To visualize regions showing significantly different FA values, significant clusters were superimposed onto SPM2’s spatially normalized template brain.
Results
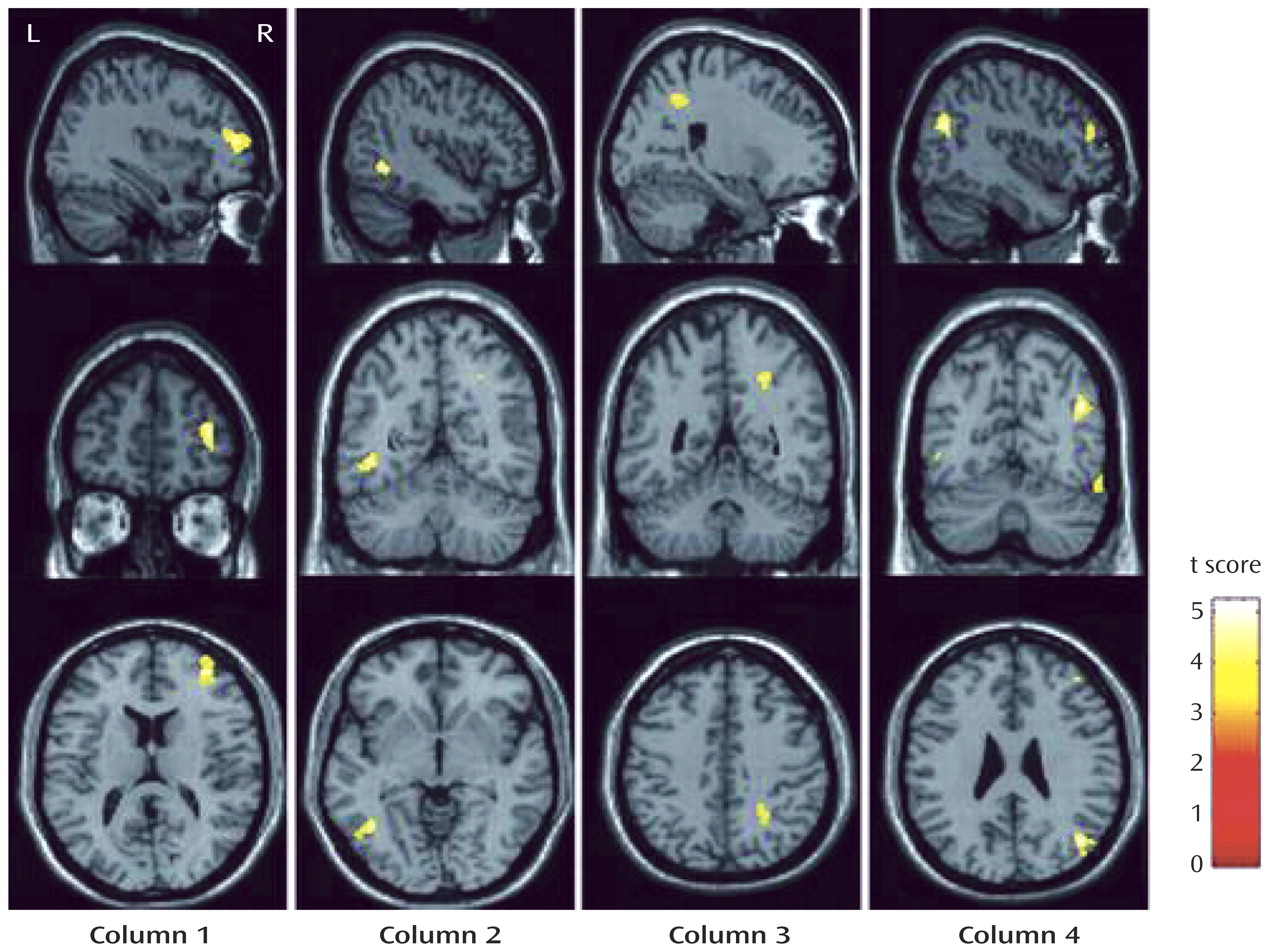
In a voxel-by-voxel contrast, compared with healthy comparison subjects, patients with major depressive disorder showed significantly lower FA values in four brain white matter regions (
Figure 1 ): the right middle frontal gyrus, the left lateral occipitotemporal gyrus, the subgyral white matter of the right parietal lobe (near the sulcus callosomarginalis and partially in the precuneus), and the right angular gyrus. The Talairach coordinates for the peak voxel in the four brain regions, respectively, were x=36, y=49, z=10; x=–42, y= –56, z=–1; x=24, y=–47, z=41; and x=42, y=–65, z=27. All four regions showed large effect sizes (1.918, 1.961, 1.789, and 2.051, respectively), as calculated with the equation d=2t/√(df)
(25) . Compared with healthy comparison subjects, patients with major depressive disorder did not show significantly higher FA values in any brain regions. Furthermore, no brain areas with significantly different FA values were found between patients with short (N=8) and long (N=6) illness durations (cutoff at 12 months), or between those with low (N=6) and high (N=8) BDI scores (cutoff at 38).
Discussion
Previous diffusion tensor imaging studies using region-of-interest methods with elderly depressed patients found white matter abnormalities in widespread frontal brain regions
(14 –
17) . Additionally, Bell-McGinty et al.
(8) reported smaller white matter volumes in the right middle frontal gyrus in geriatric depressed patients than in healthy comparison subjects by voxel-based morphometry. Our study similarly demonstrated white matter abnormalities in the right middle frontal gyrus in young adult depressed patients. The right middle frontal gyrus corresponds anatomically to the right dorsolateral prefrontal cortex, which is involved functionally in cognition and emotional regulation
(4) and has been found to show hypometabolism both in depressed subjects before treatment
(26 –
28) and in healthy comparison subjects during experimentally induced sadness
(28) . Even prefrontal lobe volume loss has been observed in late-onset and early-onset depression
(7) . Taken together, these findings suggest that loss of integrity in prefrontal white matter may exist in depression in a variety of age groups.
We observed white matter abnormalities in the left lateral occipitotemporal gyrus, which is the lateral portion of the fusiform gyrus and has extensive afferent and efferent connections with the limbic regions. Our findings are supported by two region-of-interest studies
(15,
16) that reported reduced temporal lobe white matter FA values in depressed patients and by neuroimaging studies that reported hypometabolism
(29) and abnormal neural activity
(30) in the left fusiform gyrus in depression. We also found abnormalities in the right parietal white matter among depressed subjects, which is congruent with previous studies using positron emission tomography
(28,
31), although this finding is inconsistent with that reported by Nobuhara et al.
(15) . The small sample size in our pilot study might have contributed to such differences. In addition, many other factors, including the mean age of subjects, age at onset of depression, illness duration, depression severity, drug administration, and methods of imaging data processing and analysis, could contribute to the inconsistent findings. Taken together, however, these findings support the theory that white matter lesions disrupt the neural circuits involved in mood regulation, which would contribute to the pathogenesis of major depressive disorder.
To our knowledge, this study is the first to use voxel-based analysis to explore the integrity of whole-brain white matter microstructure in first-episode, treatment-naive young adult patients with major depressive disorder. Its principal limitations are the relatively small sample size, the large range of illness duration (3–24 months), and the risk of a type I error (uncorrected). Significant clusters in our pilot study would not survive after false discovery rate correction. Furthermore, compared with the region-of-interest method, the voxel-based method cannot focus on specific brain regions and calculate concrete means and standard deviations of FA values in each cluster. Despite the limitations, using the voxel-based method with a relatively strict restriction of p<0.001 and a cluster size >50 voxels, we present evidence for possible loss of integrity of white matter fiber tracts in frontal, temporal, and parietal lobes in first-episode, treatment-naive young adult patients with major depressive disorder, which suggests that white matter pathology may occur early in the course of illness. Future studies can use diffusion tensor imaging to explore whether such abnormalities are progressive over the course of major depressive disorder as well as the relationship between these abnormalities and depression severity, response to treatment, and risk of relapse.