Scope and Nature of Neuroleptic Malignant Syndrome
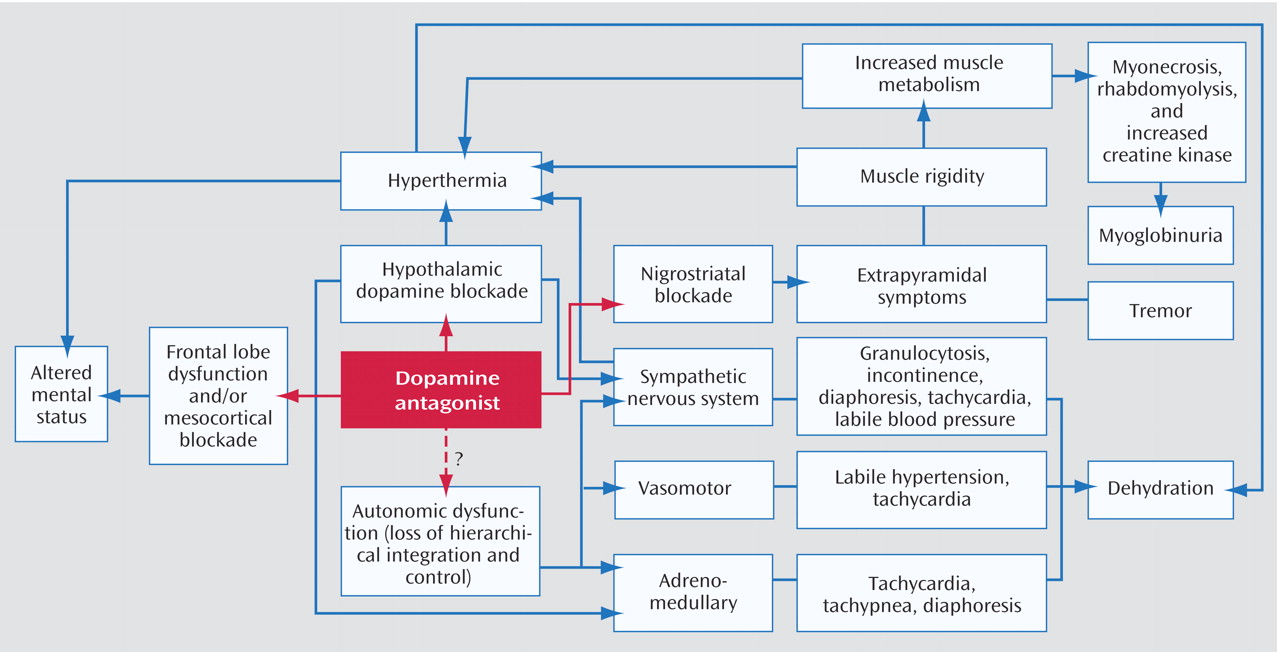
NMS, first described nearly five decades ago, is an idiosyncratic, life-threatening complication of treatment with antipsychotic drugs that is characterized by fever, severe muscle rigidity, and autonomic and mental status changes
(1,
2) . Although estimates of the incidence of NMS once ran as high as 3% of patients treated with antipsychotics, more recent data suggest an incidence of 0.01%–0.02%
(3) . This decrease in frequency likely reflects increased awareness of the disorder, more conservative prescribing patterns, and the shift to use of atypical antipsychotics. In addition, progression to more fulminant, lethal NMS episodes may occur less often because of widespread recognition and earlier diagnosis of this drug-induced reaction. Despite its declining frequency, however, NMS remains a significant source of morbidity and mortality among patients receiving antipsychotics. For example, data from the U.S. Agency for Healthcare Research and Quality indicate that about 2,000 cases of NMS are diagnosed annually in hospitals in the United States, incurring health care costs of $70 million, with a mortality rate of 10%, which underscores the continuing public health impact of NMS (http://hcup.ahrq.gov/HCUPnet.asp).
Diagnosis
Despite the availability of operational criteria
(4,
5), NMS is often difficult to distinguish from more common extrapyramidal side effects of antipsychotics and from other disorders presenting with similar symptoms
(6 –
8) . DSM-IV-TR research criteria require that both severe muscle rigidity and elevated temperature be present after recent administration of an antipsychotic as well as two associated signs, symptoms, or laboratory findings that are not better accounted for by a substance-induced, neurological, or general medical condition. Rating scales have been introduced for tracking the clinical course of NMS on the basis of factors such as severity of hyperthermia, rigidity, mental status alteration, and elevation in serum creatine kinase
(9,
10) .
Laboratory investigations are essential to exclude other disorders or complications. Several laboratory abnormalities are associated with NMS, although none are specific for the diagnosis
(7,
8) . For example, patients with NMS may have rhabdomyolysis, resulting in significant increases in serum creatine kinase, aldolase, transaminases, and lactic acid dehydrogenase concentrations, with the risk of subsequent myoglobinuric renal failure. Patients may also have metabolic acidosis, hypoxia, decreased serum iron concentrations, elevated serum catecholamines, and leukocytosis, with or without left shift. Results of CSF analysis are normal in more than 95% of cases
(11) . Findings of neuroimaging studies are generally within normal limits, and electroencephalography may demonstrate generalized slowing consistent with metabolic encephalopathy
(11) .
The temporal progression of signs and symptoms may provide important clues to diagnosis and severity of illness. Retrospective analyses suggest that alteration in mental status and other neurological signs precede systemic signs in more than 80% of cases of NMS
(8,
12) . Although the initial progression of symptoms is usually insidious over days, occasional cases of NMS may have a fulminant onset within hours after drug administration. About 16% of cases of NMS develop within 24 hours after initiation of antipsychotic treatment, 66% within the first week, and virtually all cases within 30 days
(11) . It would be unusual for NMS to occur beyond 1 month after initiation of treatment unless the dose was increased or an additional antipsychotic administered. Once NMS is diagnosed and oral antipsychotic drugs are discontinued, NMS is self-limited in most cases. The mean recovery time after drug discontinuation is in the range of 7–10 days, with 63% of patients recovering within 1 week and nearly all within 30 days
(11) . However, the duration of NMS episodes may be prolonged when long-acting depot antipsychotics are implicated. In addition, there have been several reports of patients in whom residual catatonia and parkinsonism persisted for weeks after the acute metabolic symptoms of NMS resolved
(8,
13) . Clinicians should bear in mind that although NMS is striking in its classic form, the condition is heterogeneous in onset, presentation, progression, and outcome.
Differential Diagnosis
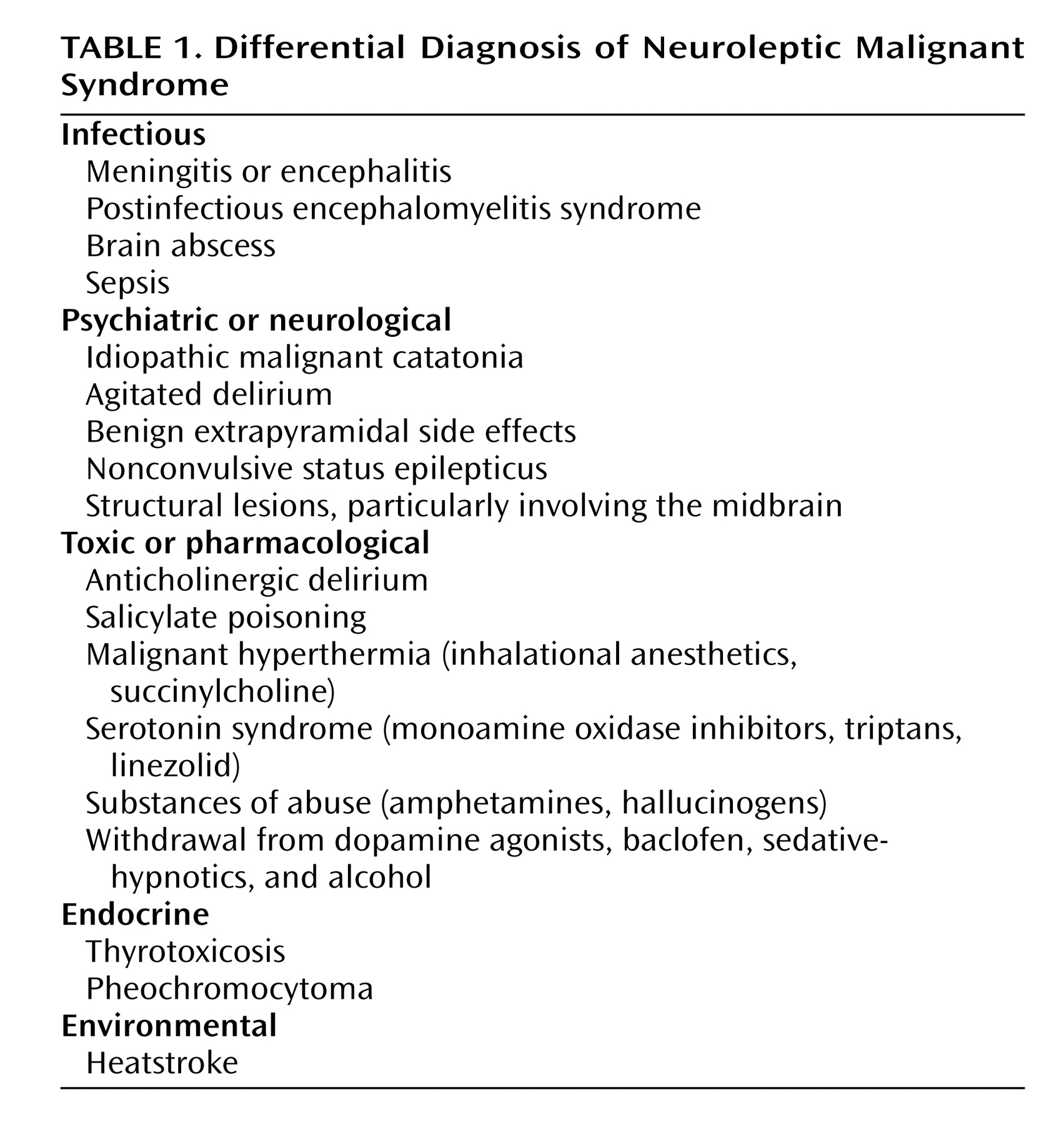
Differential diagnosis (
Table 1 ) is of prime importance because NMS is a diagnosis of exclusion. Central, systemic, and toxic causes of hyperthermia and rigidity must be excluded, as well as other causes of rhabdomyolysis and altered mental status. According to a compilation of cases reported to the Neuroleptic Malignant Syndrome Information Service, infections, agitated delirium, and benign extrapyramidal symptoms are among the processes most commonly confused with NMS (S.N. Caroff, unpublished data, 2007).
In the differential diagnosis, special attention should be given to the evaluation of CNS infections, especially viral encephalitis, which can be difficult to distinguish from NMS. Prodromal viral illnesses, headaches, meningeal signs, seizures, localizing neurological signs, CSF studies, and neuroimaging may suggest an infectious etiology. Caroff and Mann
(7) noted that the risk of severe drug-induced extrapyramidal reactions, including NMS, may be heightened in patients infected by HIV and other viruses that affect midbrain structures. Anatomic lesions affecting midbrain and brainstem structures, as well as rare cases of nonconvulsive status epilepticus, can simulate NMS and are considered in the differential diagnosis.
Advanced stages of psychotic disorders associated with excited or stuporous catatonia (delirious mania and malignant catatonia) can present with hyperthermia and appear indistinguishable from NMS
(14) . Indeed, NMS has been conceptualized as a drug-induced iatrogenic form of malignant catatonia
(14,
15) . Although some features—such as parkinsonian symptoms; extreme hyperthermia and stupor developing only after drug administration; absence of an underlying psychiatric disorder; and so on—may be suggestive of drug-induced malignant catatonia (i.e., NMS) rather than idiopathic malignant catatonia due to progression of psychotic illness, the two conditions may be indistinguishable in more than 20% of cases and may reflect the same underlying pathophysiology
(14) . In either NMS or malignant catatonia due to psychosis, antipsychotics should be discontinued; most NMS episodes are self-limited once medication is stopped, and in idiopathic malignant catatonia, antipsychotics appear to be ineffective or even detrimental. ECT appears to be the treatment of choice in malignant catatonia, and it is often effective in NMS as well.
Among systemic disorders, heatstroke can present with hyperthermia, confusion, tachycardia, and tachypnea, and its differentiation from NMS may be difficult in a psychiatric patient receiving antipsychotic medication. However, in heatstroke patients, in addition to a history of exertion or exposure to high ambient temperatures, the skin is dry and muscle flaccidity is commonly observed.
Several classes of drugs may cause symptoms resembling those of NMS. Dopamine antagonists other than antipsychotic drugs (e.g., metoclopramide, amoxapine, and prochlorperazine) have reportedly caused NMS. Withdrawal of dopaminergic agents (e.g., amantadine and l -dopa) or of the GABA-ergic drug baclofen can precipitate an NMS-like reaction. Serotonergic drugs, including selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors (including linezolid), and triptans used to treat migraine headaches, can cause serotonin syndrome, which most often presents as an agitated delirium but resembles NMS in severe cases. It is important to differentiate between serotonin syndrome and NMS not only because the treatment approaches for the two conditions may differ but also because the diagnosis will affect how one approaches resumption of antipsychotic medication in patients with persistent or recurrent psychosis.
Patients undergoing general anesthesia may develop the NMS-like signs of malignant hyperthermia. In contrast to NMS, these patients usually develop symptoms intraoperatively, have a primary pharmacogenetic skeletal muscle disorder (which consequently is not relieved by neuromuscular blocking agents), and may have a family history of malignant hyperthermia during surgery
(8,
16) .
Certain substances of abuse are associated with NMS-like presentations, among them cocaine and amphetamine (especially Ecstasy [3,4-methylenedioxymethamphetamine, or MDMA]). Hallucinogen intoxication (e.g., from phencyclidine) and withdrawal from alcohol and sedative-hypnotics also may cause fever, autonomic changes, and other symptoms that can be confused with NMS.
Risk Factors
Several studies of risk factors for NMS
(17) suggest that age, sex, and time of year are not significantly correlated with risk of developing the condition. NMS is not specific to any neuropsychiatric diagnosis, although patients with catatonia may be at risk of progressing to NMS after receiving antipsychotics.
Several clinical, systemic, and metabolic factors have been correlated with the incidence of NMS, including agitation, dehydration, restraint, preexisting abnormalities of CNS dopamine activity or receptor function, and iron deficiency
(18,
19) . Nearly all case series of NMS patients have reported physical exhaustion and dehydration prior to the onset of NMS
(17) . Elevated environmental temperature has been proposed as a contributing factor in some series, although NMS can occur independent of ambient conditions. A prior episode of NMS has been described in 15%–20% of cases
(8,
11) .
Pharmacological and treatment variables have been examined as risk factors for NMS. Nearly all dopamine antagonists have been associated with NMS, although high-potency conventional antipsychotics are associated with a greater risk compared with low-potency agents and atypical antipsychotics
(3,
7) . Parenteral routes, higher titration rates, and total dose of drug administration have been associated with an increased risk of NMS
(17) ; however, a significant number of NMS cases occur at therapeutic doses of these agents. Although cases of NMS meeting DSM-IV-TR research criteria have been reported with clozapine, olanzapine, and risperidone, unequivocal cases implicating monotherapy with quetiapine, ziprasidone, or aripiprazole remain scarce
(20) .
Although evidence from small cohort studies suggests that these clinical and pharmacological variables correlate with the risk of NMS, they are not practical in predicting risk in a given patient because they are relatively common and NMS is relatively uncommon. In other words, the association of these risk factors with NMS in a few patients may not outweigh the benefits of antipsychotics for the vast majority of psychotic patients.
Summary and Recommendations
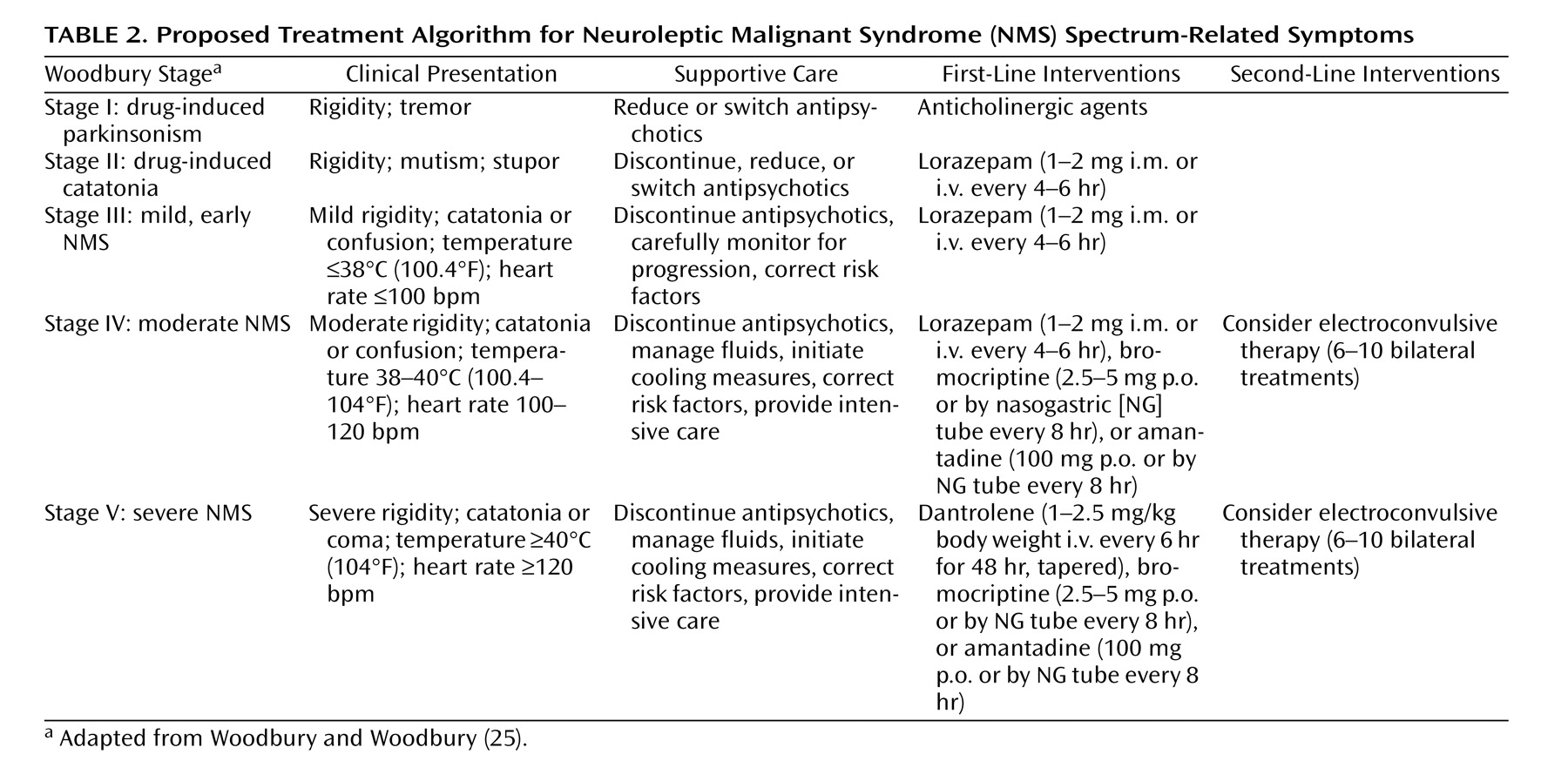
The incidence of NMS is estimated at 0.01%–0.02% of patients treated. Although the widespread adoption of atypical antipsychotics has markedly reduced the risk of neurological disorders, NMS remains a risk for susceptible patients receiving these drugs. The atypical agents are associated with less risk of NMS than the conventional antipsychotics. Nevertheless, clinicians must be aware of the clinical features of NMS and vigilant in detecting early signs. Primary management of NMS lies in prevention through conservative use of antipsychotics, reduction of risk factors, early diagnosis, prompt discontinuation of offending medications, and medical management. In the absence of randomized controlled trials, it may be unwarranted to recommend one single intervention over another or over supportive management. Specific treatment of NMS should be individualized and based empirically on the character, duration, and severity or stage of clinical signs and symptoms
(25,
26) . For mild cases, supportive care and careful clinical monitoring may be sufficient
(7,
8), whereas in severe cases, more aggressive measures should be taken, including empirical trials of specific pharmacological agents or ECT (
Table 2 ).
The patient in the vignette is suffering from severe (stage V) NMS. All antipsychotic medications should be stopped immediately, and cooling measures and aggressive medical management, including intravenous fluids, should be initiated in an intensive care setting. Lorazepam or dopaminergic agents could be tried empirically. However, given the risks of extreme hyperthermia and rigidity associated with significant rhabdomyolysis in this case, intravenous dantrolene could be administered for 48 hours, followed by tapering if the fever and rigidity resolve. If Ms. A’s symptoms do not improve after several days, ECT should be considered.
Approximately 2 weeks after resolution of NMS, treatment with a low-potency atypical antipsychotic should be initiated at a low dose and slowly titrated in a monitored setting with careful assessment for signs of recurrent NMS.