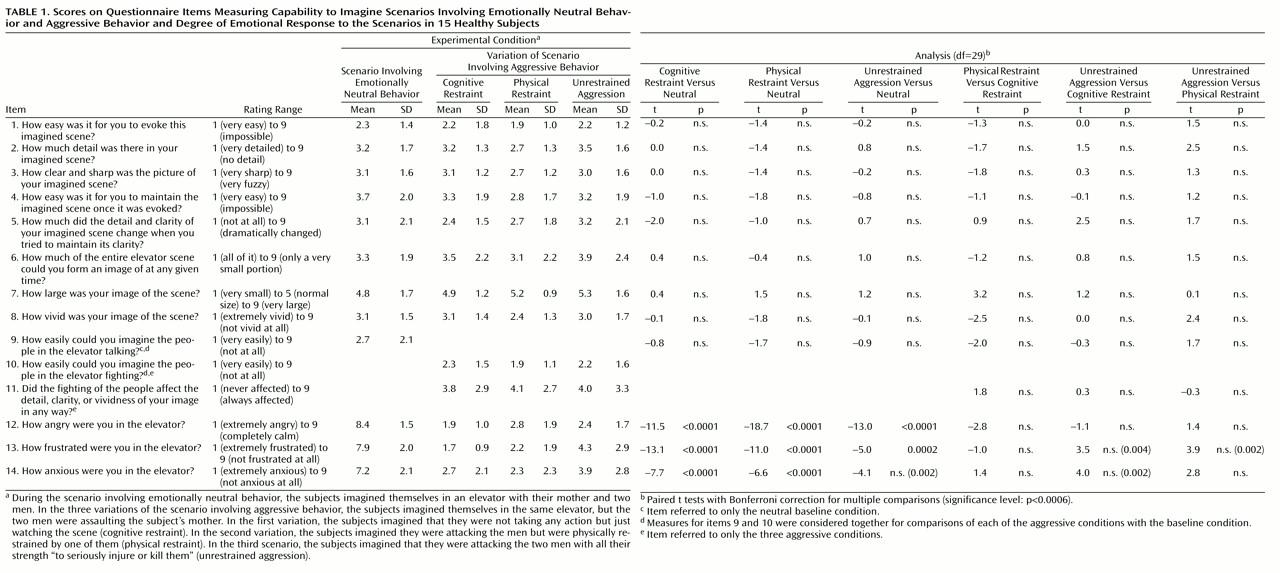
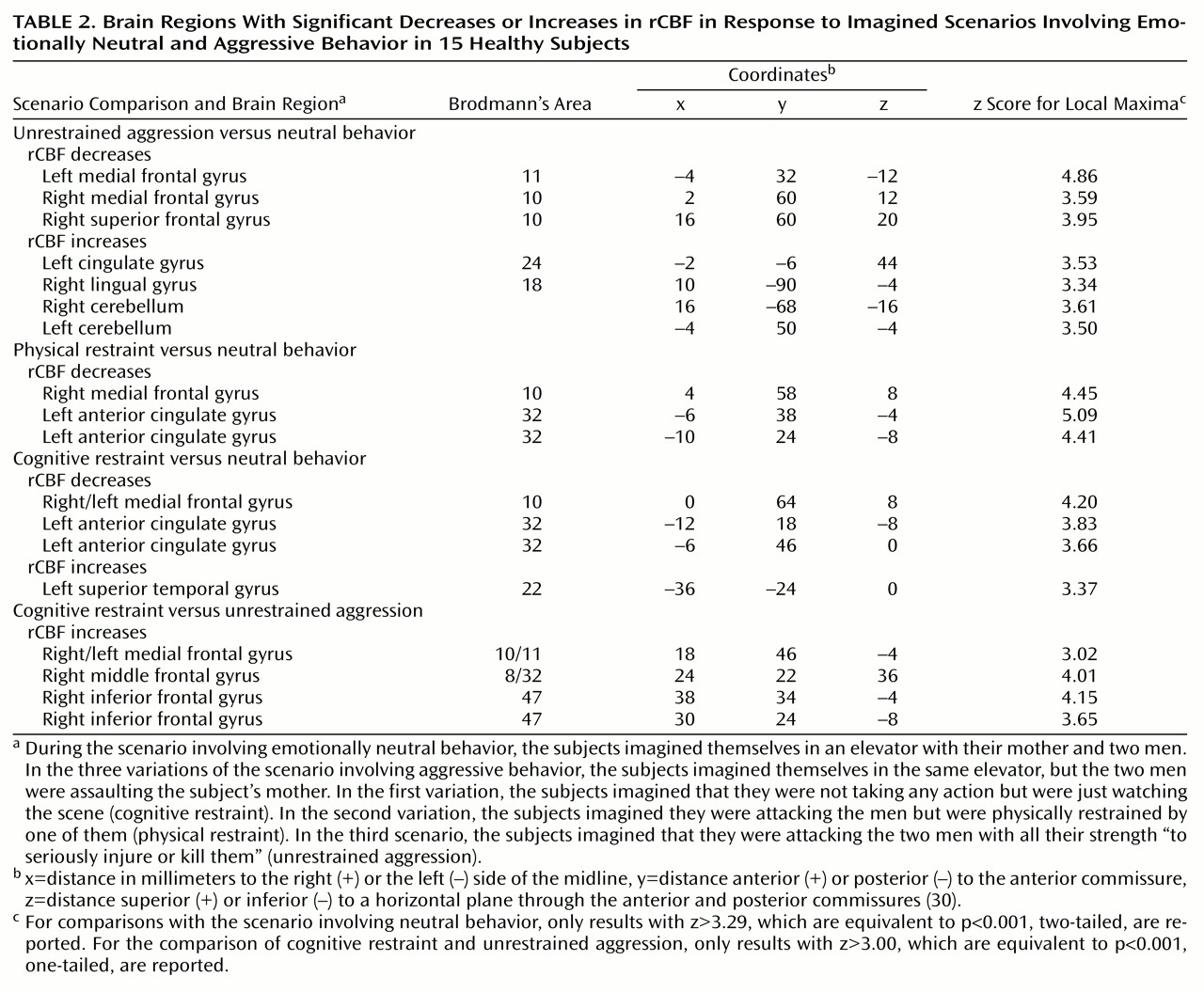
The orbitofrontal and the ventromedial frontal cortex are thought to help inhibit inappropriate social decision making and aggressive behavior
(1–
8). In humans and monkeys, stimulation of the orbital and ventromedial prefrontal cortex leads to inhibition of anger and aggression, while neurodegenerative, traumatic, or neoplastic lesions of these structures facilitate the expression of aggressive behavior and behavioral disinhibition
(5,
7,
8). In humans, positron emission tomography (PET) studies at rest have shown a lower cerebral glucose metabolism in frontal cortical regions in murderers than in nonviolent comparison subjects
(9–
11). PET studies have also shown a correlation between lower glucose utilization in the orbitofrontal cortex and history of aggressive behavior in subjects with personality disorders
(12). Although these findings have suggested a relation between dysregulation of aggressive behavior and prefrontal dysfunction, they have not informed us about the changes in brain activity that occur during the expression of aggressive behavior in healthy human subjects. We conducted a study to determine the functional role of the frontal lobes and the potential involvement of additional brain areas in the modulation of aggressive behavior. Functional brain imaging methods, including PET, have been successfully employed to explore the in vivo neural correlates of emotional behavior
(13–
18); these methods have also been used in combination with visual imagery strategies in such research
(19–
24). In the study reported here, functional neuroimaging was combined with qualitative and quantitative measures to examine brain activity and behavioral and autonomic responsiveness during the imagined evocation of aggressive behaviors in healthy volunteers with good visual imagery capabilities and without a history of mental disorders or aggression.
Discussion
Clinical observations in humans and experimental reports in primates have consistently indicated that the prefrontal cortex, and in particular the orbitofrontal region, plays a pivotal role in the regulation of social and aggressive behavior
(32–
37). Furthermore, PET studies have shown abnormal glucose utilization in prefrontal cortical areas in the resting state in violent murderers
(9,
10), in psychiatric patients with violent behavior
(12), and in association with aggressive behavior in individuals with personality disorders
(11). Recently, an average 11% reduction in gray matter of the prefrontal cortex was shown in subjects with antisocial personality disorder compared to healthy subjects
(38), further supporting the evidence that prefrontal dysfunction is associated with abnormal aggressive and violent behavior
(4–
12,
36,
39).
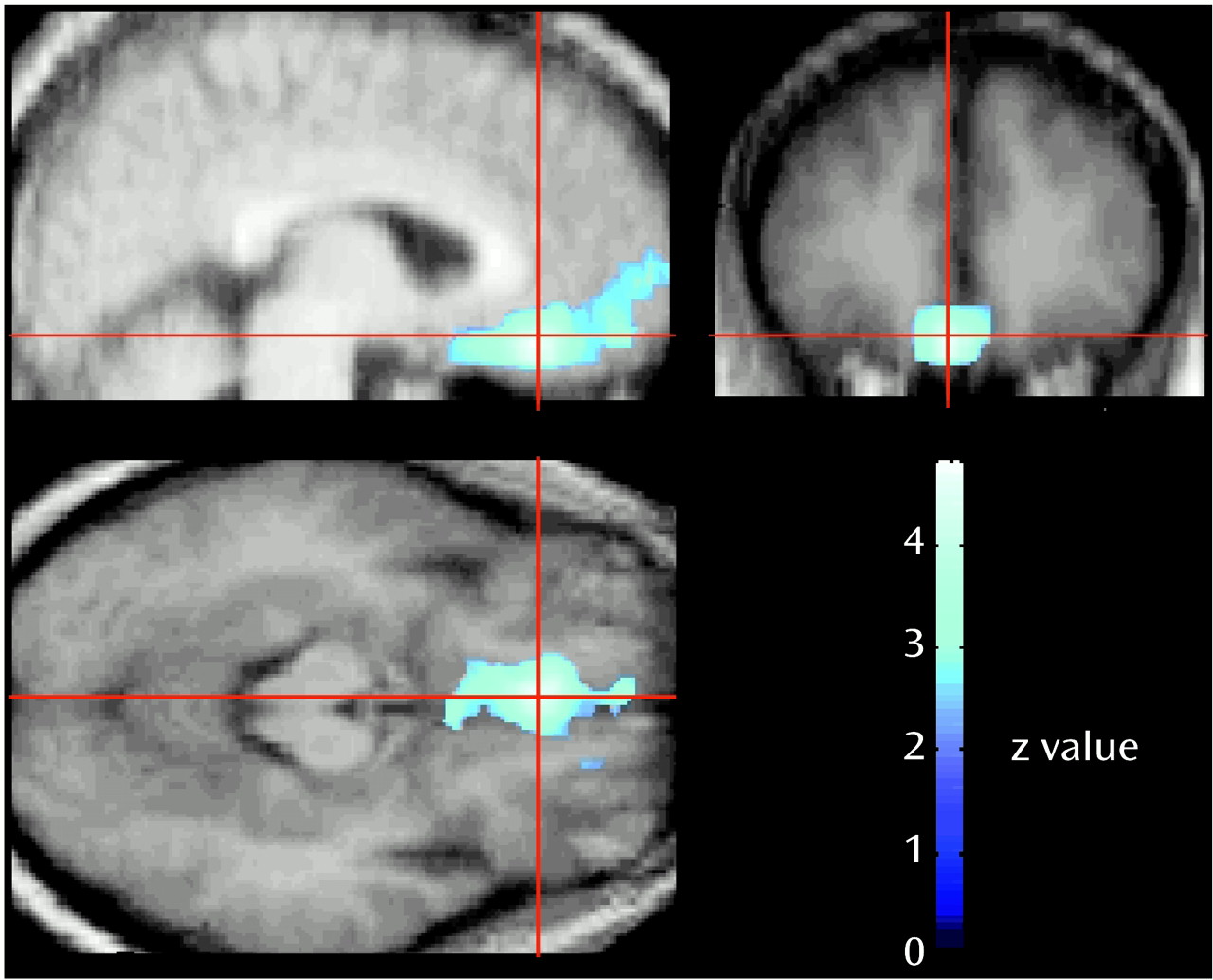
In this study, we assessed the functional brain response when healthy individuals are confronted with situations involving aggressive behavior. Consistent with our hypothesis, the results indicate that a functional decrease in the activity of the medial orbitofrontal cortex accompanies the expression of aggressive behavior even at an imaginal level. The medial orbitofrontal cortex is considered the “limbic” portion of the frontal association cortex
(32) and is intimately connected with the amygdala and the limbic system
(35,
40–
42). It is thought to play a role in integrating motivational and emotional processes
(43). It has long been known that the human orbitofrontal cortex plays a crucial role in the modulation of the expression of social and emotional behavior
(37). In rats, lesions of the orbitofrontal cortex lead to increased aggression
(44). In humans, lesions of the medial orbitofrontal cortex disrupt inhibitory and emotional mechanisms and lead to impulsive and socially inappropriate behavior
(1,
2,
33,
34,
39,
45). In a study by our group of a large cohort of patients who had received penetrating head injuries during the Vietnam War
(7), the patients with lesions of the ventromedial frontal cortex consistently showed significantly greater scores on scales and questionnaires assessing aggressive and violent behavior than did healthy comparison subjects or patients with lesions in other parts of the brain. Frontal lobe seizures are often associated with rage and aggression
(46); disinhibition of behavior, including verbal and physical aggressiveness and failure to observe social and moral rules, often is among the prominent features of degenerative processes of the frontal lobes
(47), whereas personality and behavior are virtually intact until the end stages of dementia in degenerative disorders that spare the frontal lobes
(27). Besides guiding our everyday social interactions and mediating behavior consistent with rules of socialization
(35), the modulation exercised by the orbitofrontal cortex enables humans to suppress inappropriate reactions (such as physical violence) to environmental provocations
(37). Our results are in agreement with the findings from recent PET studies that demonstrated involvement of the orbitofrontal cortex in response to anger
(22,
48). In one of those studies
(22), induction of anger in healthy male subjects through script-driven imagery resulted in activation of the left orbitofrontal cortex (including the orbital gyrus of Brodmann’s area 11 and the medial inferior frontal gyrus of Brodmann’s areas 10 and 47), compared to an emotionally neutral baseline. The authors proposed that this part of the orbitofrontal cortex was activated to inhibit an aggressive behavioral response elicited by the induction of anger. In the other study, Blair and colleagues
(48) showed enhanced activity in the right orbitofrontal cortex in response to angry facial expressions that correlated with expression intensity. Considering that angry expressions are displayed to curtail the socially inappropriate behavior of others and that the orbitofrontal cortex is involved in the mediation of behavioral extinction
(49), the authors suggested that the orbitofrontal cortex activation in response to stimuli featuring angry expressions acts to suppress current inappropriate behavior. This suppression may occur either through an inhibition of current behavior or by activation of alternative behavioral patterns
(48).
The role of orbitofrontal and prefrontal cortical areas in the evaluation of distinct negative and positive emotional situations, including sadness, happiness, fear, disgust, aversive gustatory stimulation, anticipatory anxiety, and recollection of autobiographic traumatic events, has been supported by several functional brain studies in both healthy subjects and patients with psychiatric disorders (e.g., references
(13–
24,
50–
54). Further, the identification of emotional expression is impaired in patients with lesions of subcallosal and other ventromedial prefrontal regions
(55). In particular, the ventromedial prefrontal cortex is involved in making judgments on the basis of the emotional valence of stimuli
(56,
57). Thus, the medial prefrontal cortical regions could be involved in the conscious experience of emotion, including modulation and inhibition of expression of emotions or of emotionally driven behavior
(16,
22,
48). A proper modulatory role of this cortical region in the subject’s emotional state and consequent behavioral response may be needed to make personally relevant decisions
(16,
34,
58).
The experimental paradigm designed for this study included three distinct scenarios involving aggressive behavior to control for variables that could have affected the results. Specifically, the scenario involving cognitive restraint was meant to control for the potential effects of the (imagined) motor response component, which is absent in this scenario but is present in the scenario involving physical restraint and even more so in the scenario involving unrestrained aggression. Although the specific features of the three scenarios differed in some regards, all of them involved aggressive behavior. We argue that the reduced ventromedial frontal cortex activation associated with these scenarios compared to the scenario involving emotionally neutral behavior is selectively linked to the aggressive component, as indicated by the fact that reduced rCBF in the ventromedial cortex was associated with all three aggressive scenarios. Even when the subjects were instructed to inhibit the expression of aggressive behavior (cognitive restraint), they were indeed exposed to aggressive behavior and experienced an emotionally arousing response similar to that elicited by the other two aggressive scenarios (as indicated by the scale scores). However, they had to prevent themselves from reacting. This was not an easy task because it was somewhat “unnatural” and went against the natural aggressivity of the subject. On the other hand, the rCBF associated with cognitive restraint was higher than the rCBF associated with the unrestrained expression of aggression, which suggests a less profound inactivation of this cortical area when aggression is manifested in others but must be inhibited in the observer.
The functional meaning of rCBF decreases observed in PET studies may require some additional comments. rCBF in neocortical regions reflects the balance between local excitatory and inhibitory synapses. The accepted explanation for decreases in rCBF is that they represent functional deactivations and are due to a net decrease in regional synaptic metabolic demand. This interpretation is supported by both PET studies in humans and autoradiographic studies in animals that demonstrated a decrease in local cerebral glucose metabolism and blood flow in relation to the inhibition of cortical neurons
(59–
61). For instance, the intracarotid injection of the GABA
A agonist 4,5,6,7-tetrahydroisoxazolo(5,4-c) pyridin-3-ol (THIP), which induces postsynaptic neuronal inhibition by increasing the membrane conductance to Cl– and thereby enhancing the negative membrane resting potential, is associated with a dose-dependent decrease in rCBF in the cortical areas of the affected hemisphere
(61). These deactivations may be due to a decreased excitation (i.e., the deactivated area may receive less input from another brain structure) or to a local net increase in synaptic inhibition
(62,
63). Therefore, whether due to direct inhibition or a reduced excitatory input, the decreased rCBF in the frontal cortical regions shown by our subjects during the scenarios involving aggressive behavior compared to the neutral scenario can be regarded as a true index of reduction of the functional activity in these cortical areas.
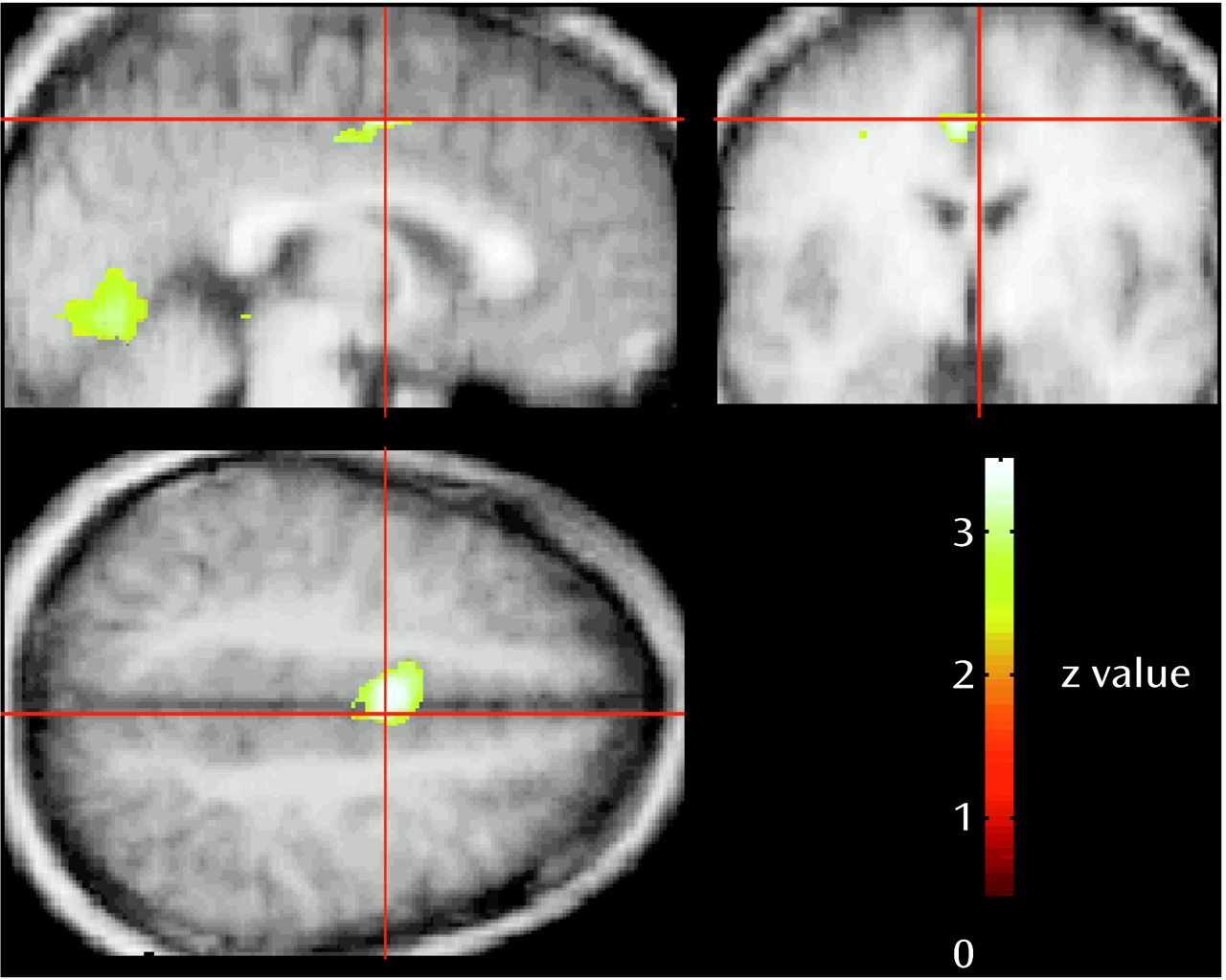
In addition to the rCBF reductions in the orbitofrontal cortex, activation of visual and limbic cortical areas and the cerebellum occurred during the scenario involving unrestrained aggression. The involvement of these regions can be explained by the distinctive features of the unrestrained aggression scenario compared to the neutral scenario. In previous studies, increased rCBF in cortical areas 17 and 18 was found while subjects visualized aversive versus neutral stimuli and during the actual viewing of emotionally aversive versus neutral pictures
(13,
14,
19,
34,
35). Thus, the higher blood flow in the visual cortical areas during unrestrained aggression may have been due to the aversive content and to greater emotional arousal and visual attention associated with this situation compared with the neutral content of the baseline condition, although no significant rCBF increase in visual cortical areas was present in the other aggressive conditions, which had similarly aversive and arousing content. Another possibility is that activation of the visual area, along with activation of the anterior cingulate cortex (Brodmann’s area 24) and of the cerebellum, may be related to the subjects’ imagining themselves actively moving while planning their “counterattacks” as well as to change in their visual perspective during the scenario involving unrestrained aggression compared with the neutral scenario or the aggressive scenarios in which they were restrained
(66–
69). Motor imagery, which can be regarded as an incomplete motor program terminated before execution
(69), led to activation of cerebellar hemispheres in several studies that used different stimulation paradigms
(66,
69–
71). It is of interest that lateral regions in the cerebellar hemispheres also were activated during imagery but not during actual motion
(69). This additional functionality during imagery may reflect inhibitory processes that terminate the motor program before execution
(72), sustained motor representations when the motor program awaits execution
(73), or elaboration of visually imagined motor actions
(71,
74). Finally, cerebellar activation may be linked to emotional processing. A growing body of evidence in the last decade has supported a cognitive and affective role for the cerebellum, in addition to its more classic role in motor function
(75,
76). Behavioral changes, including disinhibited and inappropriate behavior, are part of the so-called cerebellar cognitive affective syndrome that follows lesions of the posterior part of the cerebellum
(77). As in the case of the visual cortex, however, this interpretation cannot explain why no significant cerebellar activation was present in association with the remaining two scenarios with aggressive content, given that subjects reported a similar emotional and arousal response.
Possible gender differences in brain response are another factor that may help explain the lack of statistically significant rCBF increases in the remaining two conditions with aggressive content
(18,
78). Preliminary analyses of gender by condition in our study group showed that while rCBF decreased in relation to the aggressive scenarios in both male and female subjects, the distribution of increases in rCBF differed significantly between the two groups. Male subjects showed a significantly greater activation in visual cortical areas than female subjects, and female subjects showed a significantly greater activation in limbic structures than male subjects
(79,
80). A significantly larger activation of limbic system areas in female subjects than in male subjects has also been found during the self-induction of transient sadness
(78), despite the observation that men and women had similar abilities in self-inducing sadness. In-progress studies of gender differences in personality traits and in behavioral and brain functional response to imaginal evoking of aggressive behavior
(79,
80) will likely clarify this issue.
The magnitude and type of aggressive behavior response that can be induced during functional brain imaging studies is subject to both practical and ethical constraints. Mental imagery has been successfully employed to evaluate cognitive, behavioral, and emotional responses in previous neuropsychological and imaging studies
(18,
20–
23,
27,
65,
81–
83). Although it is impossible to determine what subjects actually do during a visual imagery scan, in this study we obtained multiple measures, in addition to rCBF changes, that indicated that subjects were engaging in mental imagery. For example, subjects’ blood pressure was significantly higher and their heart rate was higher, although not significantly so, during aggressive scenarios compared to the neutral scenario. Furthermore, all participants in this study were selected on the basis of good or excellent visual imagery abilities and were trained in the experimental procedures to minimize anxiety or discomfort associated with PET scanning and thereby to enhance compliance during the study
(84). Indeed, if the 15 subjects in the study had not engaged in the cognitive/emotional task of the distinct experimental conditions as requested, we would have had progressively increased noise in the signal and we would have not observed the significant physiological, behavioral, and rCBF results that are consistent with specific imagery that varied with experimental instructions.
In summary, although the results of this study are preliminary and should be replicated in different experimental conditions, these findings provide some initial in vivo evidence in healthy humans to support the crucial role of the orbitofrontal cortical regions in the modulation of aggressive behavior. When aggressive behavior was evoked through script-driven mental imagery, subjects showed a significant decrease in the neural activity of the orbitofrontal cortex, which is known to control the expression of aggression. These results, along with the findings from previous PET studies of baseline brain glucose utilization in subjects with abnormal behavior
(9–
12) and with data from recent structural
(38) and functional brain studies on the prefrontal cortex
(22,
48), suggest that abnormal expressions of aggressive behavior in violent individuals are accompanied by a functional “shut-down” of the orbitofrontal cortex
(37). As more sophisticated experimental paradigms are developed in conjunction with functional brain imaging methods, specific hypotheses can be tested in the investigation of the neural correlates of normal and pathological expressions of human emotions and behavior
(13–
24,
50–
54).