The introduction of clozapine, an atypical antipsychotic, for the treatment of poorly responsive schizophrenic patients has fostered widespread hope among patients, their families, and caregivers
(1–
3). For the first time in over 30 years, a treatment is available that appears to be more effective than the standard or typical neuroleptics for many such treatment-resistant schizophrenic patients.
A growing number of reports, however, suggest that clozapine may also have a role in other treatment-resistant psychotic conditions. Retrospective studies by a number of groups
(4–
10) suggest that clozapine may be particularly effective in the treatment of psychotic affective disorder, perhaps even more effective than it is for schizophrenia. Case reports
(11,
12) have further strengthened this finding.
Despite the strong suggestion of its effectiveness for patients with treatment-refractory psychotic affective disorders, there has been only one prospective study of clozapine in patients with treatment-refractory mood disorders
(13,
14). This study of 25 patients suggested beneficial effects of clozapine monotherapy over the course of a 13-week, open-label trial. We report here results of a second open-label, prospective study of clozapine monotherapy in a group of patients with refractory psychotic mania. The data from our study add to the growing evidence for efficacy of clozapine in bipolar disorder populations.
Method
Patients with treatment-refractory bipolar disorder, manic type with psychotic features, were recruited for an open-label clozapine trial from the inpatient facilities of two Harvard teaching hospitals: the Massachusetts Mental Health Center in Boston and the McLean Hospital in Belmont, Mass. Treatment-refractory was defined as a history of manic and psychotic symptoms that had not improved after a 6-week trial of 500 mg/day of chlorpromazine or its equivalent and a trial of lithium carbonate of at least 6 weeks (with a documented lithium blood level of at least 0.8 meq/liter). A documented inability to tolerate a lithium trial could substitute for nonresponse to the drug. For inclusion in this study, patients must have had a history of at least three episodes of mania in the past 2 years or a recent 6-month history of mania with psychotic symptoms with no period of remission lasting more than 2 weeks. Severity criteria at entry included a minimum score on the expanded 24-item Brief Psychiatric Rating Scale (BPRS)
(15) of 21 (using a 0–6 scale) and a Clinical Global Impressions (CGI)
(16) rating of 3 (using a 0–6 scale). Best-estimate DSM-III-R diagnoses were made after administration of the Structured Clinical Interview for DSM-III-R—Patient Version
(17) and a consensus conference. After complete description of the study to the subjects or their guardians, written informed consent was obtained.
Following withdrawal of their psychotropic medication, all patients underwent a 2–10-day drug washout period before the open-label trial of clozapine. During the washout period, treatment with lorazepam or benztropine was allowed for the control of agitation and extrapyramidal symptoms, respectively. Patients began treatment with clozapine at 25 mg/day; the dose was increased, as tolerated, by 25 mg/day to a maximum of 550 mg/day. During the clozapine trial, treatment with lorazepam was allowed if required for the control of agitation.
Ratings were performed at the end of the drug washout period and every 2 weeks thereafter over the course of the 12-week clozapine trial (six of the 22 subjects had weekly ratings). Clinical change was assessed by percent change in BPRS, Young Mania Rating Scale
(18), and CGI ratings from baseline to the final assessment point. Clinical response was defined as a 20% decrease on all three scales.
Group t tests were used to compare those who completed the study and those who did not; paired t tests were used to assess the difference in level of symptoms from baseline to the final assessment point. In order to include a subject in the data analysis, a baseline assessment and at least one postbaseline assessment were needed.
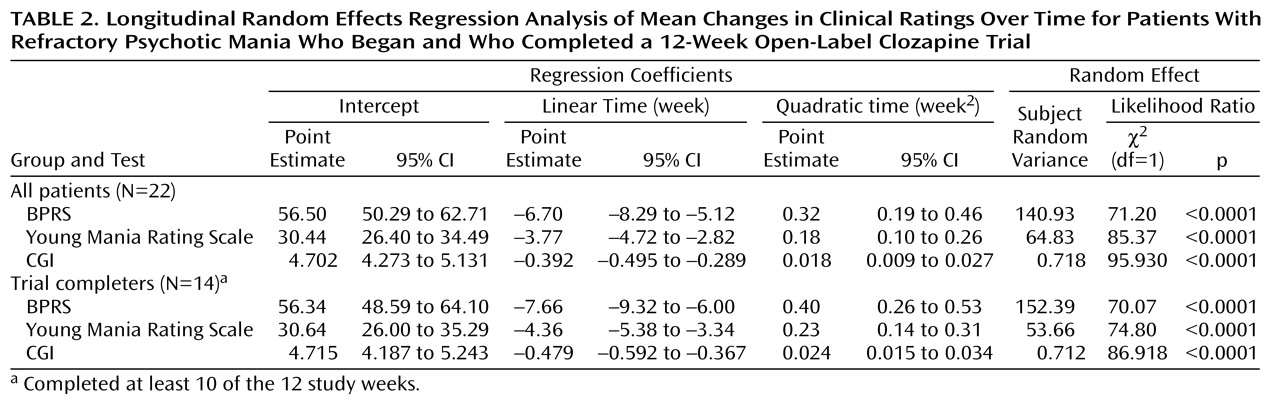
Inference about mean response to treatment over time was made by using longitudinal random effects regression analysis
(19). This approach allows use of data in which the number of measurements per subject differ, and it also reveals the baseline subject variation. The longitudinal random effects analysis provided maximum likelihood estimates of the mean rate of change in BPRS, Young Mania Rating Scale, and CGI ratings over time as quadratic functions of time.
The longitudinal random effects regression model for a subject i (i=1, 2,…22) at week j (j=0, 1, 2,…12) is expressed by a linear combination of fixed regression coefficients (β
0, β
1, β
2) and a random effect (q), i.e.,
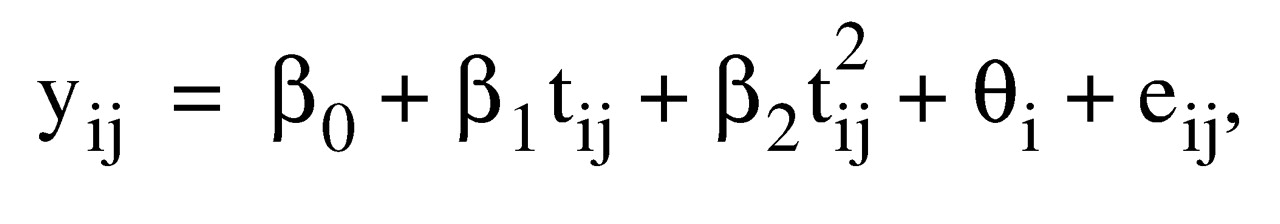
where β
0 is the fixed intercept, β
1 and β
2 are the regression coefficients for the linear (t
ij ) and quadratic (t
2 ij)time in weeks, θ
i is the measure of the effect of subject i with mean 0, and e
ij is the correlated error with mean 0. We assumed that the correlational structure of the error term e
ij has a compound symmetry, such that variances and all covariances across time points are homogeneous. The small group size did not allow a more sophisticated covariance structure.
Results
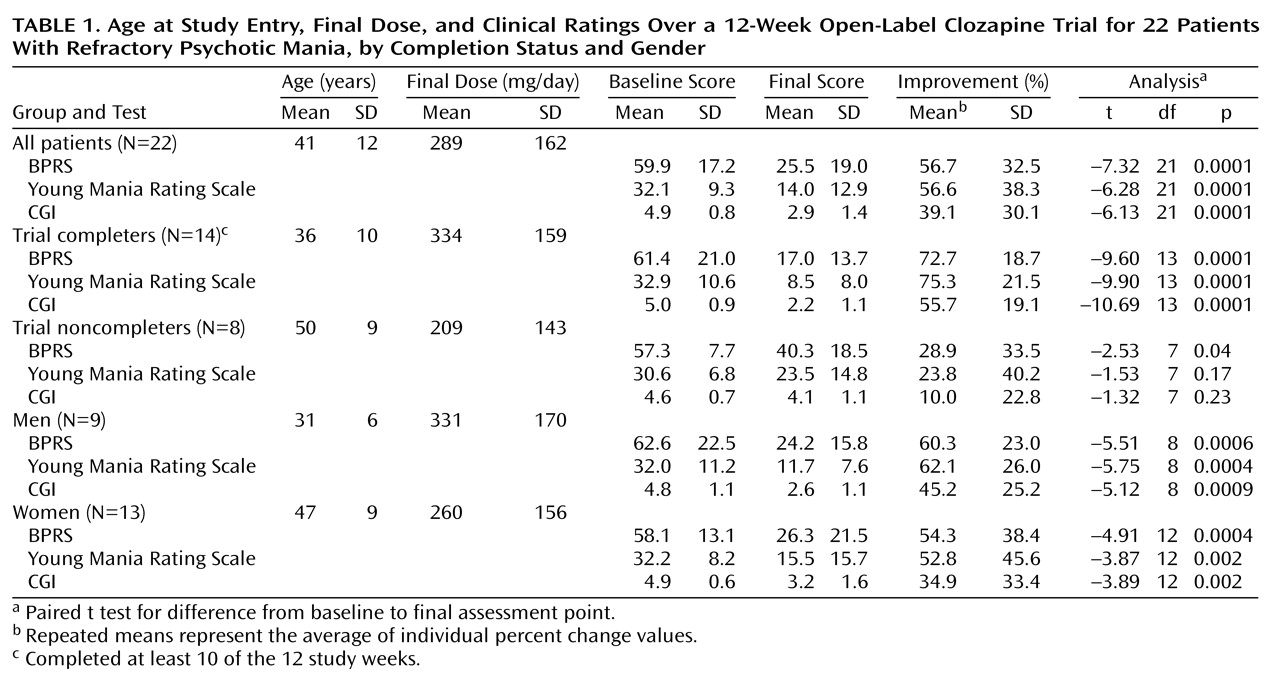
Twenty-two subjects with a diagnosis of bipolar disorder (nine men and 13 women) were included in the study. Two other subjects who provided consent were not included in the study because of missing data (i.e., no baseline clinical assessment or no postbaseline assessments.) The mean age at first diagnosis was 24 years (SD=7, range=10–44; age at first diagnosis data were not available for three subjects). The mean age at entry, final clozapine dose level, and clinical ratings over the course of the trial are presented in
Table 1.
Fourteen of the 22 patients who began the trial completed at least 10 weeks of the study and were considered “completers.” Among the eight patients who did not complete 10 weeks (“noncompleters”), seven were women. Subjects who completed the clozapine trial were younger than noncompleters (t=3.29, df=20, p=0.004) but had similar BPRS scores at baseline (t=–0.67, df=18, p=0.51) and a similar age at first diagnosis (mean=22.3 [SD=6.0] and 27.0 years [SD=8.7], respectively) (t=1.41, df=17, p=0.18). The total BPRS scores of the subjects who completed the clozapine trial were much lower at endpoint than those of the noncompleters (t=3.37, df=20, p=0.003), as were the BPRS subscale scores for positive symptoms (mean=4.9 [SD=4.9] versus 13.3 [SD=8.1]) (t=3.03, df=20, p=0.007) and negative symptoms (mean=1.4 [SD=0.9] versus 3.8 [SD=3.1], t=2.13, df=7.7, p=0.07).
The reasons for noncompletion were uncooperativeness (N=2), need for concomitant medication (N=1), depression (N=1), pneumonia (N=1), eosinophilia (N=1), fever and palpitations (N=1), and unknown (N=1). Dose escalation was limited by side effects from clozapine. Side effects reported by more than 20% of the 19 subjects for whom side-effect data were available included sedation (N=14), salivation (N=6), constipation/retention (N=6), nausea/vomiting (N=5), dizziness (N=4), and fever (N=4).
For the entire group of 22 subjects, the difference from baseline to endpoint on each scale was significant (
Table 1). The difference from baseline to the final value on each scale was also significant for the subjects who completed the clozapine trial but less for those who did not. Mean improvements of 56.7%, 56.6%, and 39.1% were seen on the BPRS, Young Mania Rating Scale, and CGI, respectively, for the entire group of subjects (N=22). Seventeen of the 22 subjects (77.3%) experienced at least a 20% improvement in scores on all three scales (the predetermined definition of clinical response). Ten subjects (45.5%) had at least a 50% improvement in scores on all three scales. For the 14 patients who completed the clozapine trial, mean improvements of 72.7%, 75.3%, and 55.7% were seen on the BPRS, Young Mania Rating Scale, and CGI, respectively. All 14 completers had at least a 20% improvement in scores on all three scales; 71.4% (N=10) of these patients had at least a 50% improvement in scores on all three scales.
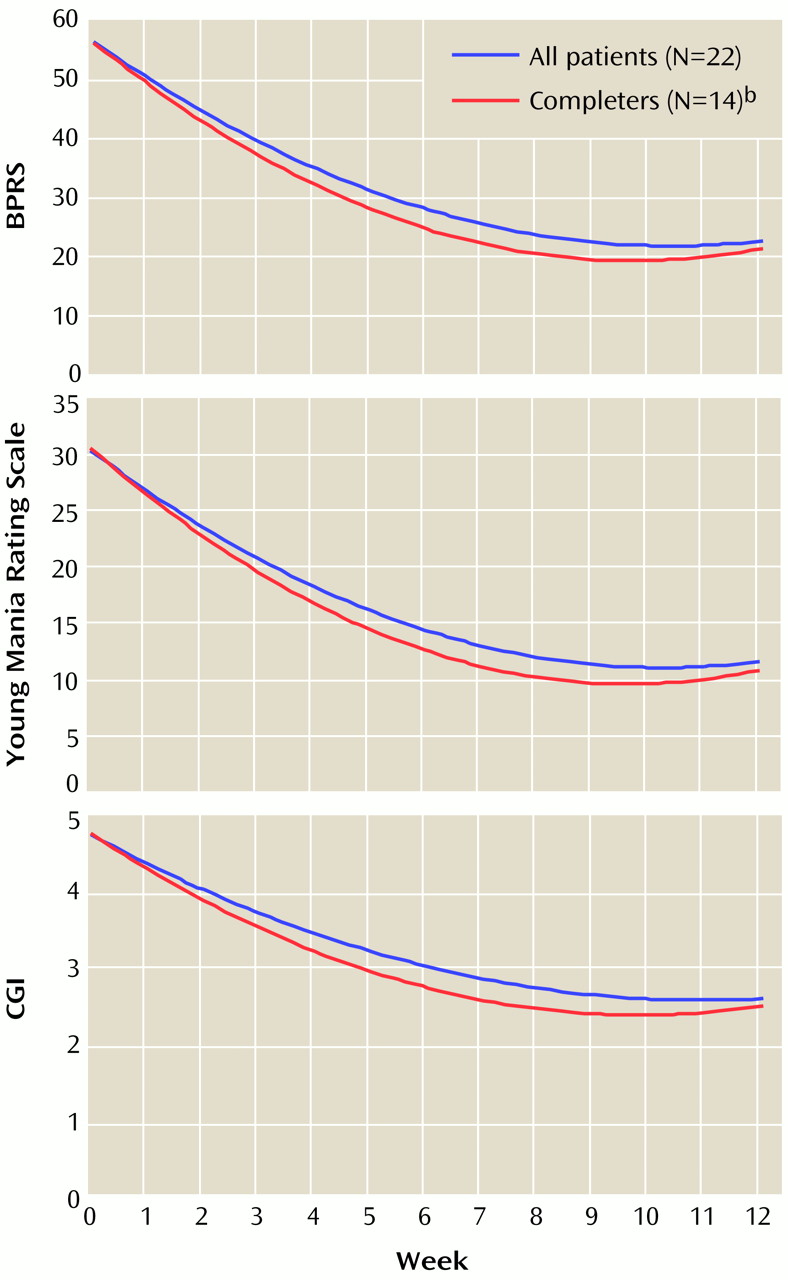
Mean changes in clinical ratings over time, determined through longitudinal random effects regression analysis, for the BPRS, Young Mania Rating Scale, and CGI for the total group of patients and for those who completed the clozapine trial are demonstrated in
Table 2 and
Figure 1. The regression coefficients shown in
Table 2 were used to draw the mean curves over time shown in
Figure 1. Most of the improvement occurred within the first 8 weeks of clozapine treatment. As evidenced by the overlapping 95% confidence intervals (CIs) of all three regression parameters between the total group and the subgroup of trial completers, there were no significant differences in ratings over time between the two groups on any of the three scales.
Discussion
While many bipolar disorder patients with psychotic mania respond well to traditional medication (including mood stabilizers and antipsychotics), a substantial portion do not respond to traditional medication
(20,
21). Our data suggest that a 12-week trial of clozapine appears to be strikingly effective as a treatment for psychotic mania in those patients who have been difficult to treat with standard agents. Our findings are consistent with previous retrospective studies
(6,
8–
10) and case reports
(11,
12) and are quite similar to the other prospective study in the literature
(13,
14). While the open-label nature of our trial calls for caution in the interpretation of these results, our data further suggest a potential role for clozapine monotherapy for this population of bipolar disorder patients.
The degree of response (on each of the clinical scales employed) was considerable. With our predetermined definition of clinical response (20% decrease in scores from baseline), we noted that 17 (77.3%) of the 22 patients had such a clinical response on all three scales. When we used a more strict definition of response (50% decrease in scores from baseline), 45.5% (N=10) of the patients met the criteria on all three scales. Not surprisingly, among the 14 subjects who were able to complete at least 10 weeks of the trial, all had at least a 20% improvement, and 71.4% (N=10) had at least a 50% improvement in scores on all three scales. Although this 3-month study did not address the issue of long-term benefit and prophylaxis against relapse, it appeared to demonstrate a considerable short-term benefit from the drug.
Eight of 22 subjects dropped out of the study before week 10 (after a mean period of 4.5 weeks in the study). Three of these eight subjects had actually exhibited treatment response at the time of their dropout—the patient who was depressed, the patient who dropped out because of the need for concomitant medications not allowed in the protocol, and the patient whose reason for dropout was not known. Although a dropout rate of 36.4% is quite high, it is possible that fewer patients would have dropped out of the trial had the procedures been more flexible clinically. On the other hand, our observation that seven of eight dropouts were women, and that the dropouts were older than the subjects who completed the trial, suggests that older women with psychotic mania may either not respond as well or may have more side effects from the drug than younger men. A preferential response of men to clozapine has been reported previously for patients with schizophrenia
(22).
Our finding that one of our patients dropped out of the study because of depression would seem to be inconsistent with case reports in the literature that have suggested that clozapine can be of value in the treatment of depressive disorders
(23–
26). Its role in bipolar depression requires further investigation.
Although the results of this study are quite striking, these data (coupled with similar data reported by other groups) suggest the need for a double-blind trial that compares both the acute and chronic effects of clozapine in the treatment of bipolar disorder patients who have not responded to conventional medications.
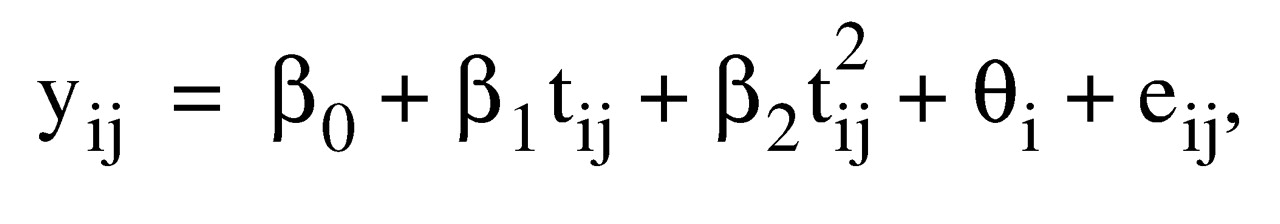 where β0 is the fixed intercept, β1 and β2 are the regression coefficients for the linear (t ij ) and quadratic (t2 ij)time in weeks, θ i is the measure of the effect of subject i with mean 0, and e ij is the correlated error with mean 0. We assumed that the correlational structure of the error term e ij has a compound symmetry, such that variances and all covariances across time points are homogeneous. The small group size did not allow a more sophisticated covariance structure.
where β0 is the fixed intercept, β1 and β2 are the regression coefficients for the linear (t ij ) and quadratic (t2 ij)time in weeks, θ i is the measure of the effect of subject i with mean 0, and e ij is the correlated error with mean 0. We assumed that the correlational structure of the error term e ij has a compound symmetry, such that variances and all covariances across time points are homogeneous. The small group size did not allow a more sophisticated covariance structure.