Schizophrenia is often characterized by deficits in adaptive functioning ranging from difficulties in performing basic activities of daily living to problems maintaining competitive employment
(1). Impairments in neurocognitive functioning are believed to underlie the problems in instrumental and role functioning observed in this illness
(1,
2). In a recent study using path analysis, we found that cognitive deficits rather than the positive or negative symptoms of schizophrenia predicted poor performance in basic activities of daily living
(3). In fact, several investigations have found that neurocognitive deficits predict approximately half of the variance in measures of adaptive functioning
(3,
4). In a comprehensive review of 17 studies, Green
(2) found measures of verbal memory, executive functions, and attention to predict multiple domains of community outcome for patients with this disorder. Furthermore, cognitive deficits have been seen as rate-limiting factors in the ability of patients to benefit from psychosocial rehabilitation
(15).
Recent studies have found that treatment with atypical antipsychotic medications can improve neurocognitive performance
(6–
8). However, even with these newer treatments, significant cognitive deficits remain
(6). Thus, the development of strategies to compensate for residual cognitive impairment is important to pursue. One promising approach is the use of compensatory strategies—environmental adaptations designed to bypass lingering neurocognitive impairments and improve adaptive functioning. Compensatory strategies include the use of signs, labels, and electronic devices designed to cue and sequence appropriate behaviors. These techniques have been used successfully for years to treat patients with head injuries and mental retardation but have only recently been applied to schizophrenia in a systematic manner
(9).
Cognitive adaptation training is a manual-driven group of compensatory strategies used to address impairments in the adaptive functioning of patients with schizophrenia. The study reported here examined the effect of cognitive adaptation training in medicated outpatients recently discharged from a state psychiatric facility. We hypothesized that rates of relapse and levels of positive and negative symptoms for patients in cognitive adaptation training would be lower at the end of 9 months than for patients in control conditions. In addition, we hypothesized that patients participating in cognitive adaptation training would have higher levels of adaptive functioning than patients participating in control conditions.
Method
Design
Forty-five patients were randomly assigned to one of the three treatment conditions: 1) standard medication follow-up, 2) standard follow-up plus cognitive adaptation training, or 3) standard follow-up plus a condition that controlled for therapist contact time and for changes in the patient’s environment. Each group included 15 patients. The treatment groups are described below. Patients in groups 2 and 3 were seen weekly for a 9-month period. Therapist contact time for these two groups was equivalent. In addition, the same individuals (bachelor’s-level psychology and social work practicum students) provided treatment for both groups. Patients were assessed on entrance into the study and at 3-month intervals throughout the study by research personnel who were unaware of subjects’ treatment groups. Patients who relapsed were assessed at the point of relapse and dropped from the study. Their last observation was used for an endpoint data analysis.
Subjects
Subjects were 45 patients recruited at discharge from a state psychiatric facility after treatment for an acute exacerbation of their psychosis. After providing written informed consent, subjects were interviewed by a master’s-level research assistant to ensure that they met the following entry criteria: 1) diagnosis of schizophrenia or schizoaffective disorder based on the Structured Clinical Interview for DSM-IV (SCID)
(10), 2) age between 18 and 55, 3) no history of seizure disorder, head trauma, organic brain disorder, or mental retardation, 4) history of compliance with antipsychotic medication and clinic visits, 5) no history of substance abuse or dependence in the past 3 months, and 6) discharge destination to an apartment, family home, or boarding facility within 70 miles of the hospital. All patients received standard follow-up care, including medications, through public outpatient clinics throughout the 9 months of the study. Medication was prescribed in doses in the recommended therapeutic range for all but two patients. One patient in the cognitive adaptation training group received 3 mg daily of risperidone (lower than the recommended dose), and one patient in the control condition received 1000 mg of clozapine daily (higher than the recommended dose).
Eighty-four percent of subjects participating in the study had a diagnosis of schizophrenia (N=38), and the remainder met criteria for schizoaffective disorder (N=7). Seventy-five percent (N=34) were men. Forty-eight percent of subjects were Mexican American (N=22), 37% were Anglo (N=17), and the remainder were African American, Asian, or of mixed ethnicity (N=6). The mean age of all subjects was 37.12 years (SD=8.99). Mean age at onset of psychosis was 22.35 years (SD=4.74). The majority of patients had at least a high school education. Socioeconomic status was in the range of lower-middle to low income. The length of the index hospitalization ranged from 4 weeks to 48 months, with a mean of 6 months (SD=9.9).
Treatment Groups
Cognitive adaptation training group
Cognitive adaptation training is a manual-driven series of compensatory strategies based on neuropsychological, behavioral, and occupational therapy principles
(9). Cognitive adaptation training procedures include a comprehensive behavioral assessment utilizing the Frontal Lobe Personality Scale
(11) to quantify the level of apathy and disinhibition in overt behavior. Furthermore, a neuropsychological assessment is conducted to examine the level of executive functioning (i.e., problem-solving, cognitive flexibility, and the ability to plan and carry out goal-directed activity), attention, and memory. Adaptive functioning is assessed by using the Functional Needs Assessment
(12), which identifies specific areas of impairment in activities of daily living. Finally, an environmental assessment is conducted in the patient’s home environment to identify triggers that may promote maladaptive behavior, the presence of safety hazards, the availability of needed equipment or supplies, and the organization of belongings. These assessments have been described in detail elsewhere
(9).
Treatment plans that include cognitive adaptation training are based on two dimensions: 1) the patient’s level of apathy versus disinhibition, and 2) the patient’s level of impairment in executive functions. Behaviors characterized by apathy can be altered by providing prompting and cueing that help the patient initiate each step in a sequenced task. Examples of environmental alterations for apathetic behavior include: using checklists for tasks that involve complex behavioral sequencing, placing signs and equipment for daily activities directly in front of the patient (e.g., placing a toothbrush, toothpaste, and a sign summarizing steps in brushing teeth in a basket attached to the bathroom mirror), using labels, and using electronic devices (e.g., tape recorders and menu-driven electronic cooking instructions) to cue and sequence behavior. Individuals who exhibit disinhibited behavior respond well to the removal of distracting stimuli and behavioral triggers and to redirection. For disinhibited behavior, supplies are organized to minimize inappropriate use (e.g., placing complete outfits with one shirt, one pair of pants, etc., in separate boxes in the patient’s closet to prevent him or her from putting on multiple layers of clothing; providing different colored bins for sorting laundry to help prevent patients from mixing clean and soiled clothing). Individuals with mixed behavior (both apathy and disinhibition) are offered a combination of these strategies.
Individuals with greater degrees of executive impairment are provided a greater level of structure and assistance and more obvious environmental cues (larger, more brightly colored, and more proximally placed cues). Individuals with less impairment in executive function can perform instrumental skills adequately with less structure and more subtle cues. These general plans are adapted for individual strengths or limitations in verbal/visual attention, memory, and fine motor coordination. For example, the color of signs may be changed frequently to capture the individual’s attention, or Velcro may be used instead of buttons on clothing to help individuals with fine-motor problems. Interventions are explained and maintained or altered as necessary by means of brief weekly visits from a cognitive adaptation training therapist.
Control group
The control condition was designed to account for some of the nonspecific effects of cognitive adaptation training. Subjects assigned to this condition were seen on the same schedule as those assigned to cognitive adaptation training and were given adaptations for their environment that were unrelated to cognitive or adaptive functioning (e.g., posters, plants). They were allowed to choose two items per month. Contact time was equivalent to that in the cognitive adaptation training group, and the same therapists provided treatment for both the cognitive adaptation training and control conditions.
Follow-up only group
Subjects assigned to this condition were assessed on the same schedule as those in the other two treatment conditions, but they did not receive any additional interventions besides standard follow-up care.
Assessments
Symptoms
The expanded version of the Brief Psychiatric Rating Scale
(13) is a 24-item scale that is used for assessing a wide range of psychopathology on a series of Likert-type scales from 1 to 7. The psychosis factor score, composed of items assessing hallucinations, unusual thought content, suspiciousness, and conceptual disorganization, was used as a measure of positive symptoms. Higher scores indicate higher levels of symptoms.
Negative symptoms were assessed by using the Negative Symptom Assessment
(14). The Negative Symptom Assessment is a 26-item scale assessing multiple domains of negative symptoms, including communication, social behavior, emotion, motivation, cognition, and psychomotor retardation. Higher scores indicate more negative symptoms. As described by Eckert et al.
(15), a total score for the Negative Symptom Assessment, calculated by determining the mean of the six subscales, was used as a measure of negative symptoms. The motivation subscale of the Negative Symptom Assessment was used to assess involvement in productive activities. Increased involvement in productive activity is a particular focus of cognitive adaptation training.
Global functioning was assessed by using the Global Assessment of Functioning scale (DSM-IV). This instrument assesses the overall level of functionality on a scale from 1 to 100 on the basis of the clinician’s judgment about the patient’s social and occupational functioning and the impact of symptoms on functionality. Higher scores indicate better adaptive functioning. Midway through the study we added an additional, more comprehensive measure of functionality, the Multnomah Community Ability Scale
(16). The Multnomah Community Ability Scale is a 17-item instrument that is rated by the clinician on the basis of an interview with the patient. To increase the validity of ratings, collateral information was obtained from caregivers and relatives. The total score on the Multnomah Community Ability Scale reflects the overall level of community functioning; higher scores indicate better functioning. The numbers of subjects in each group are smaller than 15 for analyses that include this variable.
Relapse
Relapse was considered to have occurred if the patient was rehospitalized during the study or if the patient experienced a significant exacerbation of positive symptoms, defined as an increase of 2 points or more to a score of 4 or greater on at least two of the four BPRS items composing the positive symptom subscale. In all but two cases of relapse, both of these criteria were met. In one of those cases, the relapse of a patient in the control condition was characterized by a return of hallucinations and delusions. However, the patient was not hospitalized due to an intense effort by family members to provide 24-hour supervision. In the second case, the evidence for the relapse of a patient in the cognitive adaptation training condition consisted of suicidal behavior rather than a worsening of psychosis.
Data Analysis
Group differences in symptoms and functionality at the end of 9 months were examined by using a series of analyses of covariance (ANCOVAs). For each variable, scores on assessments obtained at study entry were used as covariates. We plotted residuals versus predicted scores and residuals versus the covariate for each variable to verify that the assumptions for the model were correct. For each variable, we verified that the slopes of the covariate were the same for the three groups. On one measure (the Multnomah Community Ability Scale), where the variances between groups were unequal, we verified that after adjusting for the covariate, the residuals from the model had homogeneous variances for the three groups. We examined planned comparisons between the cognitive adaptation training condition and each of the two remaining conditions (control and follow-up only) by using Dunnett’s procedure to correct for experiment-wise error rate. In each comparison, the means at the end of the study, corrected for the covariate, were compared.
In addition to endpoint analyses (with the last observation used as endpoint) described above, we did an additional series of repeated measures analyses of variance (ANOVAs). These ANOVAs were conducted by using the SAS GLM procedure
(17) on only the data collected in an effort to examine the time (a within-subjects factor) at which groups (a between-subjects factor) began to diverge with respect to dependent variables. (These analyses were adjusted for time points for which data were missing on some subjects.) We examined planned comparisons of the interaction of group and time between the cognitive adaptation training group and the remaining groups by using Dunnett’s procedure at each time point.
Results
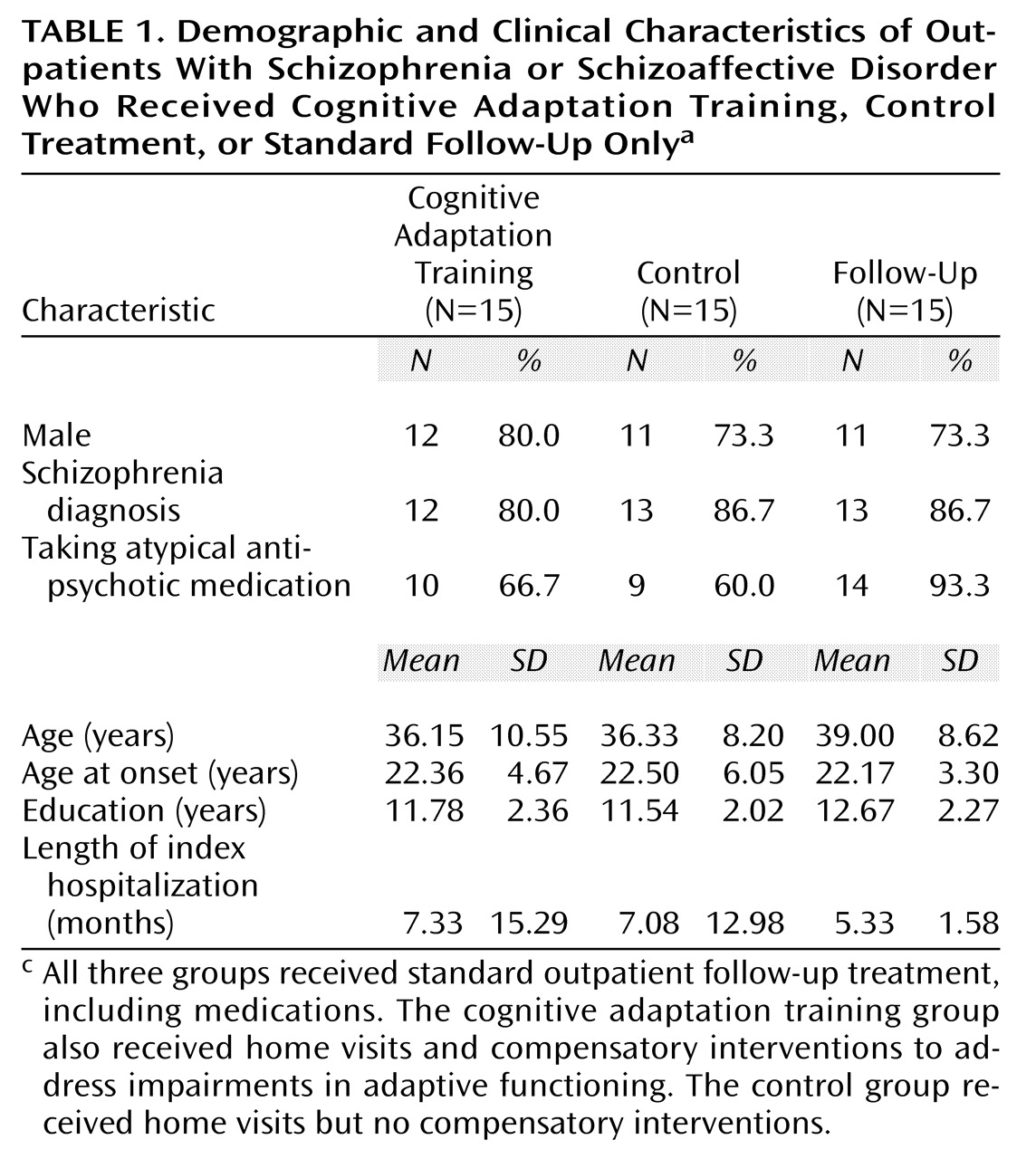
The demographic and clinical characteristics of the three treatment groups are presented in
Table 1. There were no statistically significant differences between groups on any of these variables at the time of initial assessment. However, 14 of 15 subjects in the follow-up only group (93.3%), but only 9 of 15 subjects in the control group (60.0%), were taking atypical antipsychotic medications (χ
2=4.0, df=1, p<0.07). Ten of 15 subjects in the cognitive adaptation training group (66.7%) were taking atypical antipsychotic medications.
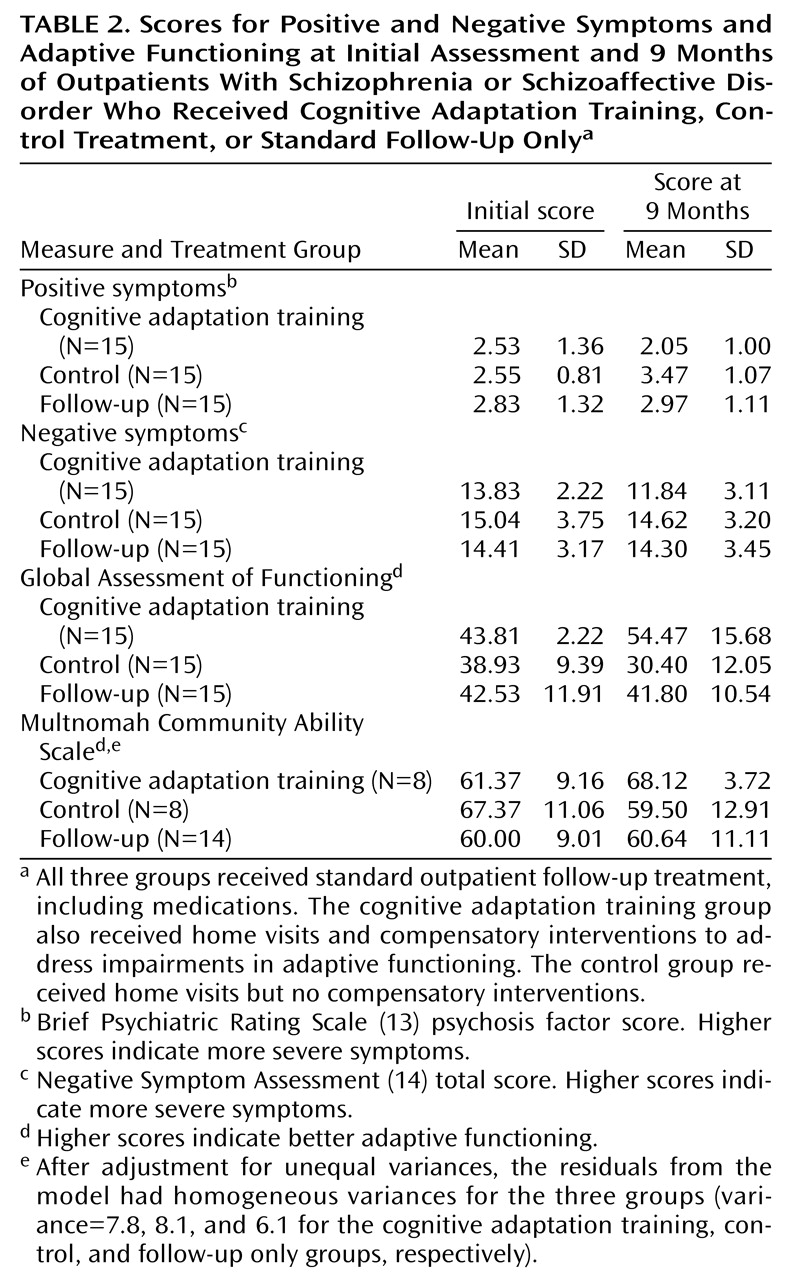
The three treatment groups’ mean scores and standard deviations on measures of symptoms and adaptive functioning at the initial and final assessments are presented in
Table 2.
Symptom scores
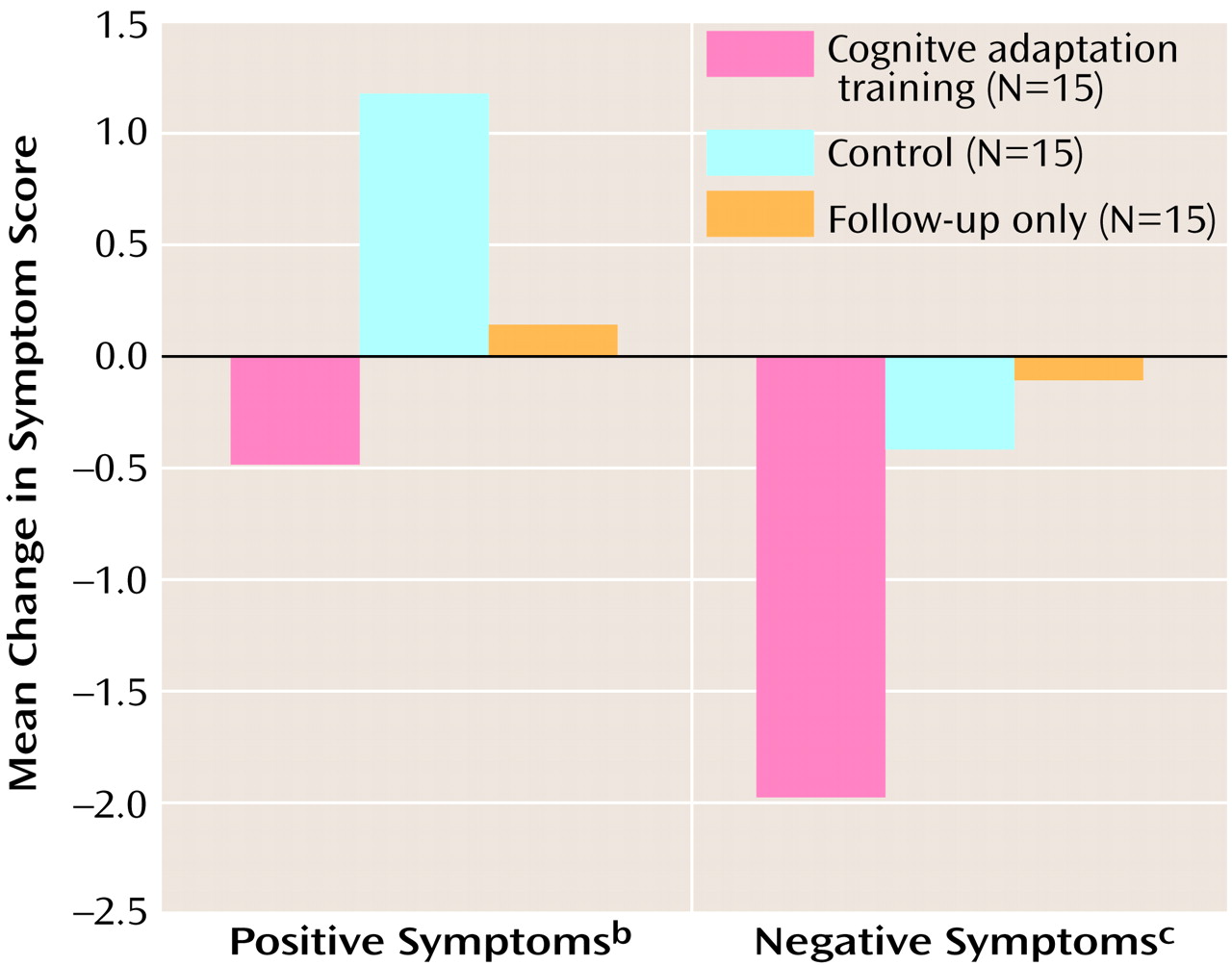
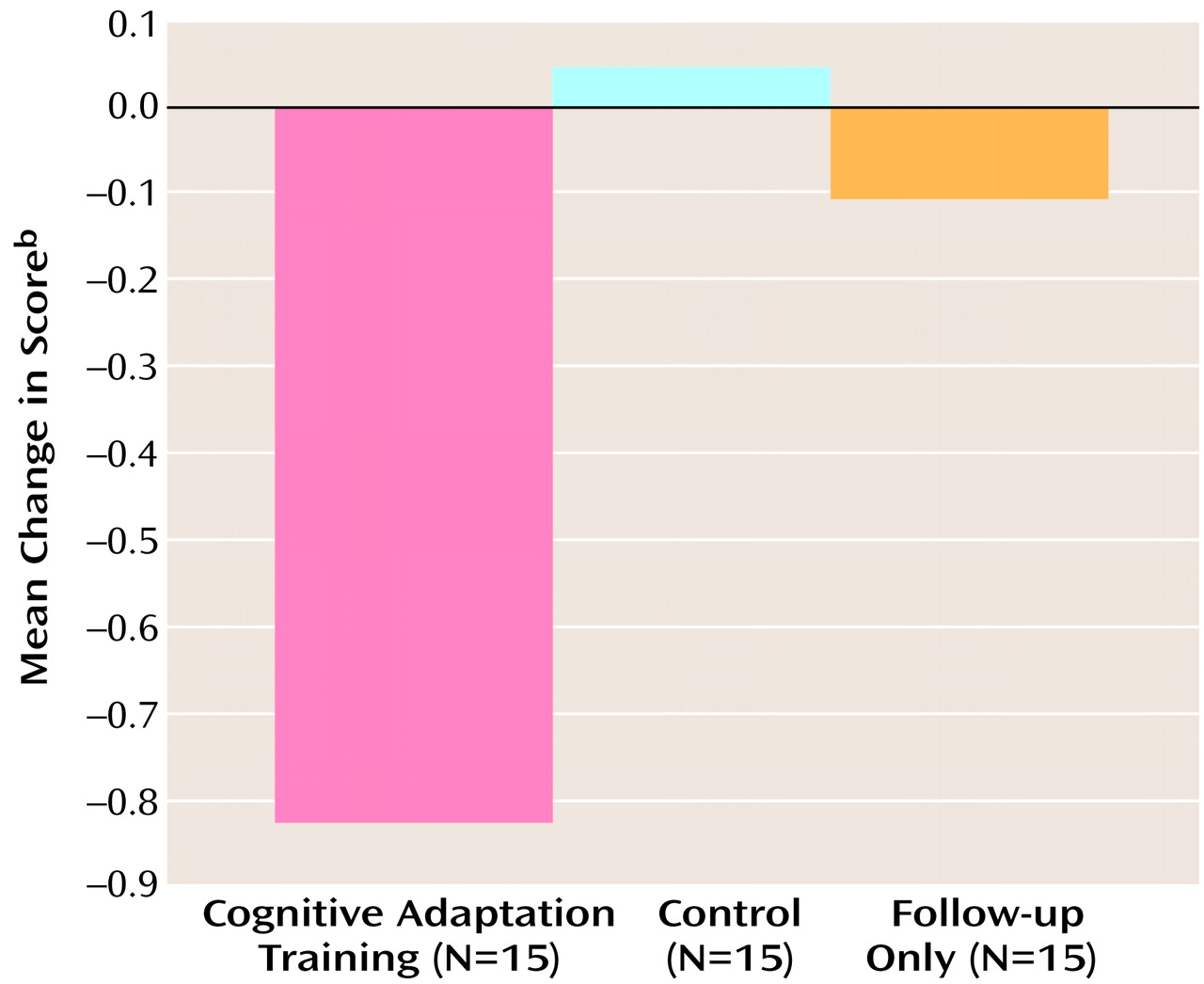
ANCOVA revealed a significant difference among the three treatment groups in positive symptom scores at the end of the 9-month study period (F=8.31, df=2, 41, p<0.001). Planned comparisons were conducted to examine differences between the cognitive adaptation training group and the two remaining groups (control and follow-up only). Only the mean difference in positive symptom scores between the cognitive adaptation training group and the control group was statistically significant, when the analysis was corrected for multiple comparisons. An inspection of means indicated improvement of symptoms in the cognitive adaptation training group and worsening in the other treatment groups. Differences in positive symptom scores between the initial and final assessments for the three treatment groups are presented in
Figure 1.
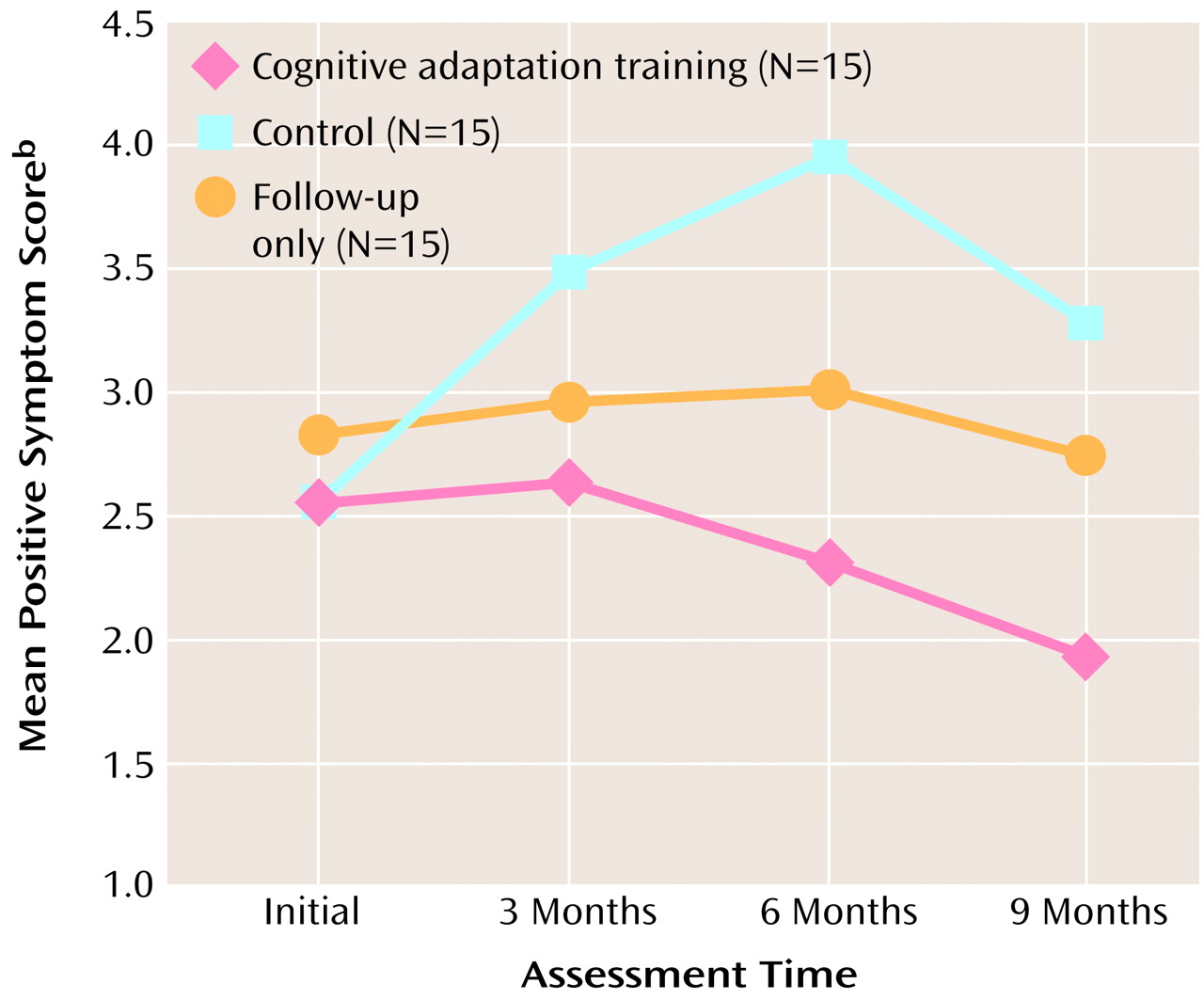
Repeated measures ANOVA for positive symptoms revealed a nonsignificant main effect for group (F=2.59, df=2, 42, p<0.09), a significant main effect for time (F=3.38, df=3, 100, p<0.02), and a significant interaction of group and time (F=2.89, df=6, 100, p<0.02). Significant differences in positive symptom scores between the cognitive adaptation training group and the control group emerged at 3 months and continued throughout the follow-up period. Differences between the cognitive adaptation training group and the follow-up only group were significant at 9 months. Mean positive symptom scores by group and assessment period are presented in
Figure 2.
With respect to negative symptom scores, ANCOVA results showed no significant difference between the three treatment groups at the end of the 9-month study period (F=2.73, df=2, 41, p<0.08). Differences in negative symptom scores between the initial and final assessments for the three treatment groups are presented in
Figure 1.
As for motivation subscale scores, the ANCOVA results showed significant differences among the three treatment groups (F=6.78, df=2, 41, p<0.003). In addition, planned comparisons that used Dunnett’s procedure to correct for multiple comparisons showed that the differences between the cognitive adaptation training group and both the control group and follow-up only group were statistically significant. An inspection of means indicated that the motivation problems of patients in the cognitive adaptation training group decreased to a greater extent than those of patients in other treatment conditions. Differences in motivation scores between the initial and final assessments for the three treatment groups are presented in
Figure 3.
Repeated measures ANOVA for the total Negative Symptom Assessment score revealed a nonsignificant main effect for group (F=2.69, df=2, 42, p<0.08), a significant main effect for time (F=2.82, df=3, 100, p<0.05), and a significant interaction of group and time (F=3.47, df=6, 100, p<0.005). Comparisons between the cognitive adaptation training group and the two remaining treatment groups at each time period, with corrections for multiple comparisons, revealed that the negative symptom scores of the cognitive adaptation training group were significantly different from those of the control group at 3 and 6 months. At 9 months, the negative symptom scores of the cognitive adaptation training group differed significantly from those of the follow-up only group. Results for the motivation subscale from the Negative Symptom Assessment were more consistent. Significant main effects were found for group (F=4.69, df=2, 42, p<0.02) and time (F=4.87, df=3, 100, p<0.004), and there was a significant interaction of group and time (F=3.17, df=6, 100, p<0.007). Significant differences in motivation were apparent between the cognitive adaptation training group and the control group by 3 months and remained significant throughout the study. Significant differences between the cognitive adaptation training group and the follow-up only group appeared at the 9-month assessment. In the interest of space, these data are not presented in figure form.
Level of Functioning
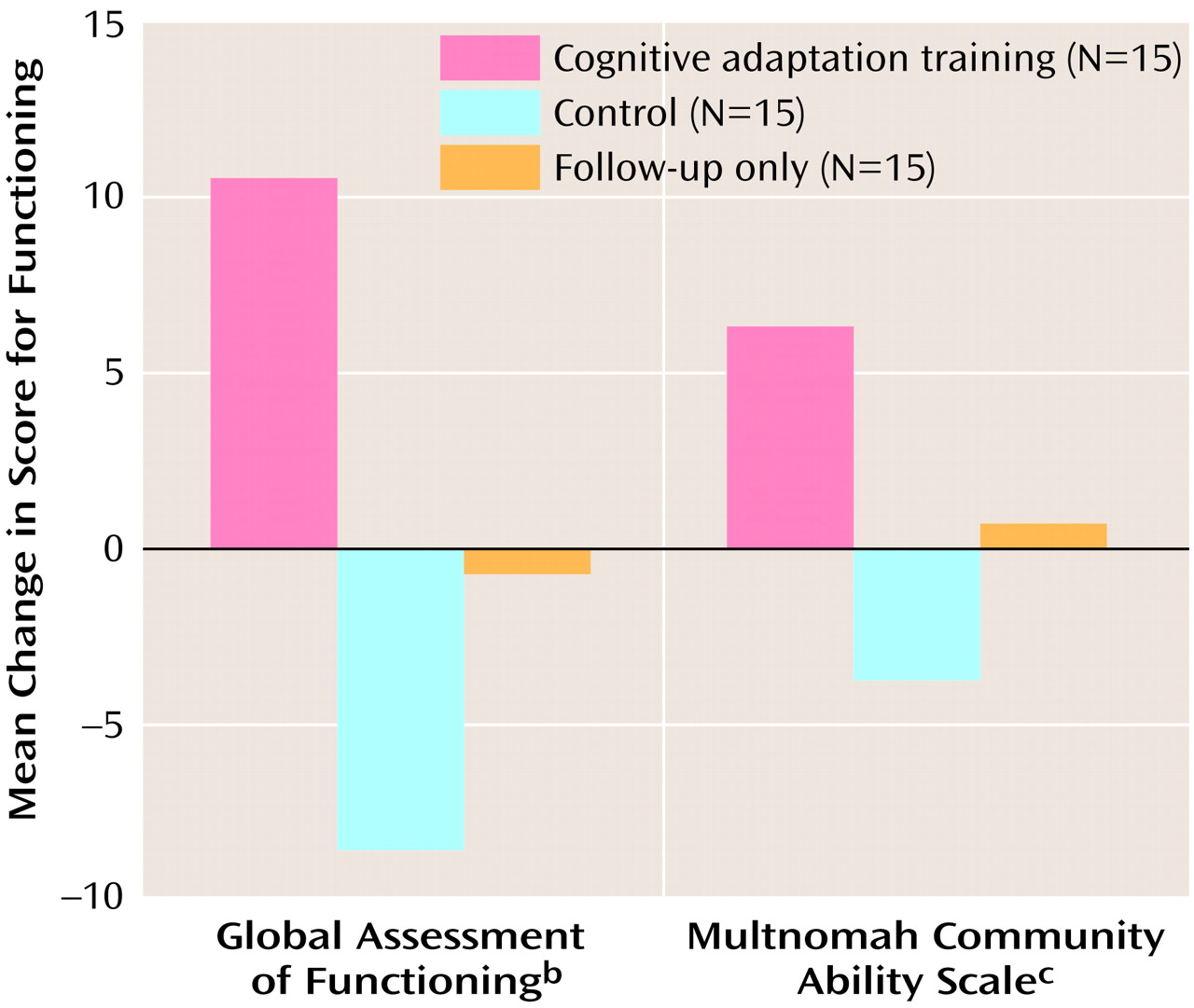
We used an ANCOVA, with the initial Global Assessment of Functioning score as a covariate, to examine whether Global Assessment of Functioning scores differed between groups at the end of treatment. The results indicated a main effect for treatment group (F=13.12, df=2, 44, p<0.0001). In addition, planned comparisons that used Dunnett’s procedure showed that the score for the cognitive adaptation training group was significantly different from the score for both the control group and the follow-up only group. Differences in scores for level of functioning between the initial and final assessments for the three treatment groups are presented in
Figure 4.
With respect to individual subjects, scores on the Global Assessment of Functioning indicated that all but two of the 15 subjects in the cognitive adaptation training group improved in level of adaptive functioning over the 9-month study period. In the control group, 12 of 15 individuals got worse or experienced no change in adaptive functioning. Finally, in the follow-up only group, 10 of 15 individuals got worse or experienced no change in adaptive functioning.
Results of a repeated measures ANOVA for the Global Assessment of Functioning score indicated a significant main effect for group (F=7.71, df=2, 42, p<0.002), a nonsignificant main effect for time (F=2.63, df=3, 100, p<0.06), and a significant interaction of group and time (F=3.87, df=6, 100, p<0.002). Differences between the cognitive adaptation training group and the control group were significant at 3 months and were sustained throughout the study. Significant differences between the cognitive adaptation training group and the follow-up only group appeared at 9 months.
An ANCOVA indicated that the scores on the Multnomah Community Ability Scale of the three treatment groups were significantly different (F=4.46, df=2, 26, p<0.02). (Eight patients in the cognitive adaptation training group, eight patients in the control group, and 14 patients in the follow-up only group participated in this assessment.) The results of planned comparisons between the cognitive adaptation training group and both the control group and the follow-up only group were significant after the analysis corrected for multiple comparisons. An inspection of means indicated a clinically significant improvement of 10 points in the cognitive adaptation training group, compared to a worsening of symptoms and almost no change in the control group and the follow-up only group, respectively (
Figure 4).
The results of the repeated measures ANOVA for scores on the Multnomah Community Ability Scale were essentially the same as those for Global Assessment of Functioning scores.
Relapse rates
Number of relapses by treatment group was examined by using chi-square analysis. Relapse rates for the cognitive adaptation training, control, and follow-up groups were 13.33%, 67.67%, and 33.33%, respectively. The difference between groups was significant (χ2=9.27, df=2, p<0.01).
Discussion
This is the first randomized, controlled study we are aware of that has demonstrated the benefit of compensatory strategies for outpatients with schizophrenia. Although these strategies have been used successfully for patients with other disorders, they have not been applied in a systematic way to the treatment of patients with schizophrenia.
Patients who received cognitive adaptation training did better than those in the control and follow-up only conditions with respect to level of symptoms and level of adaptive functioning. Improvements in functioning seen in patients participating in cognitive adaptation training were not observed in the vast majority of patients assigned to other treatment conditions. In addition, relapse rates were better for those participating in cognitive adaptation training. Compensatory strategies appear to help individuals with their transition from inpatient status to living in the community.
The control condition, which included a therapist’s weekly visits to patients and manipulation of the environment in nonspecific ways, did not lead to better outcomes. In fact, patients in the control condition did worse than those in the follow-up only group. We examined whether the poor outcomes for individuals in the control condition relative to the follow-up only group may have been due to the almost universal use of atypical antipsychotic medications in the follow-up only group. When we examined data for only those patients in each group who were taking atypical antipsychotic medications, the results were unchanged.
An alternative explanation for the difference may be that whenever the therapists who made the home visits observed problems that needed the attention of clinic staff, they asked patients to contact their clinic or case manager. These contacts may have resulted in a higher level of intervention (and accompanying clinic chart documentation) for patients in the control condition. Because these patients were seen every week, any serious problems they experienced were more likely to be identified, compared with those of patients in the follow-up only group. However, the therapists who visited the subjects in the cognitive adaptation training group and the control group, as well as the raters who assessed the subjects, did not make determinations about the need for hospitalization but rather referred subjects to clinic treatment team members who were blind to the subjects’ treatment groups.
Differences in rates of medication compliance between the groups could possibly explain some of the differences in symptom scores between the cognitive adaptation training group and the other treatment groups. Although we selected patients who were compliant with medication treatment and we had evidence from chart review that the subjects had regularly attended clinic appointments as outpatients before their index hospitalization, these factors may not guarantee that medication compliance was equivalent between groups. However, all patients had a history of willingness to take medications. If patients in the cognitive adaptation training group had better compliance, it may have been due to the compensatory strategies used in cognitive adaptation training to cue patients to take medications at appropriate times. This result would support the use of compensatory strategies. Previous research has indicated that medications are not sufficient for improving functional outcomes in patients with schizophrenia
(18). It is therefore somewhat unlikely that differences in medication compliance alone would have produced differences in levels of functioning between groups.
Additional research will be needed to examine whether cognitive adaptation training is as effective as other currently available treatments, such as Assertive Community Treatment teams. Certainly, compensatory strategies could be used by Assertive Community Treatment teams to help focus interventions.
The study reported here has several methodological weaknesses, including a small sample size and the lack of a therapeutically active control condition. These limitations will need to be addressed in future studies. In addition, the subjects included only patients who were recently hospitalized in a state hospital. The results may not apply to more stable outpatients. However, in an ongoing study with stable outpatients, preliminary results have been similar to those presented here
(19).
Despite the limitations mentioned above, the results of this study suggest that the use of compensatory strategies may add to the growing repertoire of interventions that can help patients with schizophrenia and schizoaffective disorder lead more productive and satisfying lives. Continued development and study of compensatory strategies for this population may identify which approaches are best for which patients.