Twin, family, and adoption studies have consistently suggested a genetic contribution to the etiology of schizophrenia
(1). Excitatory amino acid neurotransmission may be involved in the etiology of schizophrenia. The most compelling link between glutamatergic neurotransmission and schizophrenia is related to the mechanism of action of the psychotomimetic phencyclidine (PCP)
(2). PCP and other
N-methyl-
d-aspartate (NMDA) receptor antagonists, such as ketamine and MK-801, produce a psychotic condition, probably due to their blockade of NMDA receptors
(3). The ability of the NMDA receptor antagonists to induce a syndrome closely resembling schizophrenia suggests that dysfunction or dysregulation of NMDA receptor-mediated neurotransmission occurs in schizophrenia.
The NMDA receptor is thought to be formed from different combinations of the NMDA receptor 1 (NR1) subunit and one of the NMDA receptor 2 (NR2A-D) subunits. NMDA receptor heterogeneity may result from differential assembly of homologous NMDA receptor subunits. In the rat brain, the NR2B subunit was detected almost ubiquitously at birth, but during the first 3 postnatal weeks, the NR2B subunit became confined to anterior forebrain structures
(4). Dysregulation in such transition of the distribution of the NR2B subunit in the brain may be involved in the etiology of schizophrenia, consistent with the neurodevelopmental hypothesis for the origins of the disorder
(5).
The cloning of a cDNA encoding the human NR2B subunit has been described
(6). The NR2B subunit is equipped with an unusually long calboxyl-terminal domain that is believed to extend into the cytoplasm. The NR2B subunit is the major tyrosine-phosphorylation protein in the postsynaptic density fraction
(7), which contains enriched signal-transduction molecules regulating receptor localization and function in the brain. For example, a-actinin-2, a member of the actin-binding group of proteins, binds to the cytoplasmic tail of the NR2B subunit
(8). A major phosphorylation site of the NR2B subunit by Ca
2+/calmodulin-dependent protein kinase II has been located in the carboxyl-terminal domain
(9). These lines of evidence suggest that the intracellular carboxyl-terminal domain of the NR2B subunit plays a crucial role in cellular signal transduction.
In the study reported here, we screened for genetic variations in the human NR2B subunit encoding the carboxyl-terminal intracellular domain, which might contribute to schizophrenia. To investigate a possible contribution of this polymorphism to the etiology of schizophrenia, we compared frequencies of genetic variations in unrelated patients with schizophrenia and a healthy comparison group.
Method
This study was approved by the ethics committee of Kobe University School of Medicine. Written informed consent was obtained from all subjects. The study group consisted of 164 unrelated Japanese patients who met DSM-IV criteria for schizophrenia (105 men and 59 women; mean age=51.5 years, SD=14.1). The comparison group consisted of 171 unrelated healthy volunteers (104 men and 67 women; mean age=47.8 years, SD=14.0).
The deduced amino acid sequence of the human NR2B subunit gene has an overall identity of 90% with the reported mouse sequence
(10). Adopting the gene structure topology proposed for the region encoding the carboxyl-terminal domain of the mouse NMDA receptor e3 subunit (NR2C subunit in human)
(11), which consists of a small-sized exon (26 amino acids) and a large-sized exon (378 amino acids), that region of the human NR2B subunit appears to consist mostly of a large-sized exon. Seven sets of polymerase chain reaction primers were prepared to produce approximately 300 base-pair overlapping fragments covering the complete deduced large-sized exon. Standard polymerase chain reaction was performed, and 22 patients with schizophrenia were screened by using single-strand conformation polymorphism analysis. The DNA fragments displaying a different pattern during single-strand conformation polymorphism analysis were subjected to direct sequencing. An endonuclease
Aci I was used to confirm a mutation identified by polymerase chain reaction direct sequencing and to carry out an analysis of the presence of the mutation in the patients with schizophrenia and in the comparison group.
Differences in the allele and genotype frequencies between the patients with schizophrenia and the comparison group were tested for significance using the chi-square test.
Results
The fragment size of each polymerase chain reaction product obtained by the procedure mentioned above coincided with the expected size. We examined the mutations in the large-sized exon encoding the carboxyl-terminal intracellular domain of the human NR2B subunit. One point mutation was identified by single-strand conformation polymorphism and sequence analysis. This mutation was located at cDNA nucleotide position 2664, and was silent, changing codon 888 from ACC to ACT. We failed to detect other mutations that changed the amino acid sequence in the examined region.
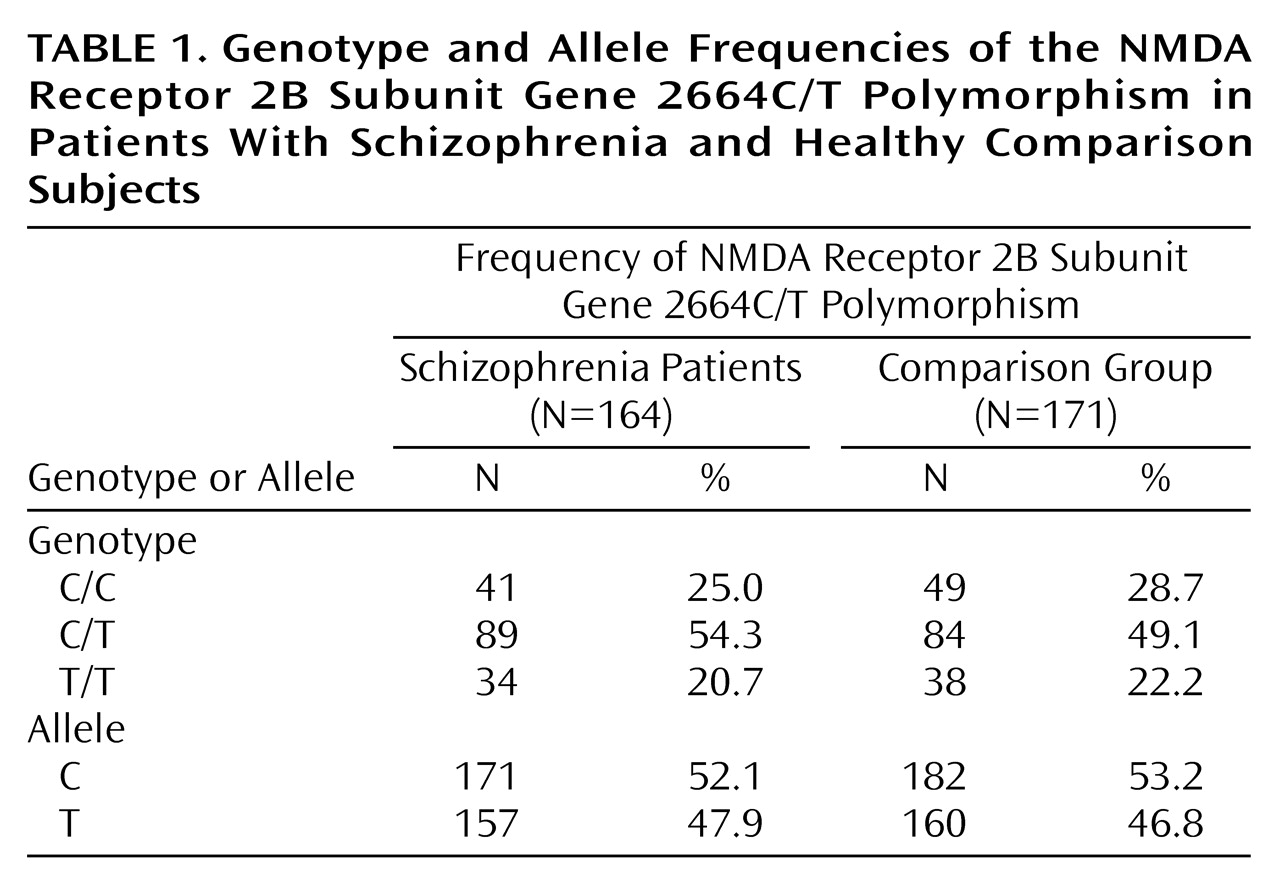
Table 1 shows the distribution of genotype and allele frequencies of the 2664C/T polymorphism for the patients with schizophrenia and the comparison group. The genotype and allele frequencies in the patients with schizophrenia did not differ from those in the comparison group (genotype, χ
2=0.93, df=2, p>0.10; allele, χ
2=0.08, df=1, p>0.10).
Discussion
We found one nucleotide sequence variant (2664C/T) in the region encoding the carboxyl-terminal intracellular domain of the NR2B subunit. This report is the first we are aware of to describe a polymorphism in the human NR2B subunit gene.
Based on our data indicating no association between schizophrenia and the 2664C/T polymorphism, it is unlikely that the human NR2B subunit gene contributes to the etiology of schizophrenia. However, NMDA receptors mediate neuronal signaling, affect neuronal gene expression, and are involved in neuronal plasticity, outgrowth, and survival. Especially, the human NR2B mRNA distribution is restricted to the frontoparietotemporal cortex and hippocampus
(10), which are the sites that are probably affected in schizophrenia. Therefore, the NR2B gene is still a possible candidate for association with schizophrenia. There may be polymorphisms in the NR2B subunit gene that cause alternations in protein function, and such alternations in function could contribute to the etiology of schizophrenia. Further attempts are needed to find genetic markers and/or functional mutations at sites of the NR2B subunit gene other than the region encoding the carboxyl-terminal intracellular domain and to determine whether such polymorphisms, if they exist, are associated with schizophrenia.