The extremes of manic excitement, pressure of speech, grandiosity, and lack of judgment can appear as profound, but transient, disturbances in the behavior of otherwise well-organized and successful individuals. It seems unequivocal that disturbance of brain function underpins these characteristics of mania. Neuropsychological paradigms offer fresh insight into the links between symptoms, elemental cognitive functions, and the underlying neural substrates of abnormal mental states. If we assume a core abnormality in mania, then there should be consistent effects on some functional domains and perhaps more variable effects on neuropsychological functions that are less closely yoked to the core disturbance of mood and activation.
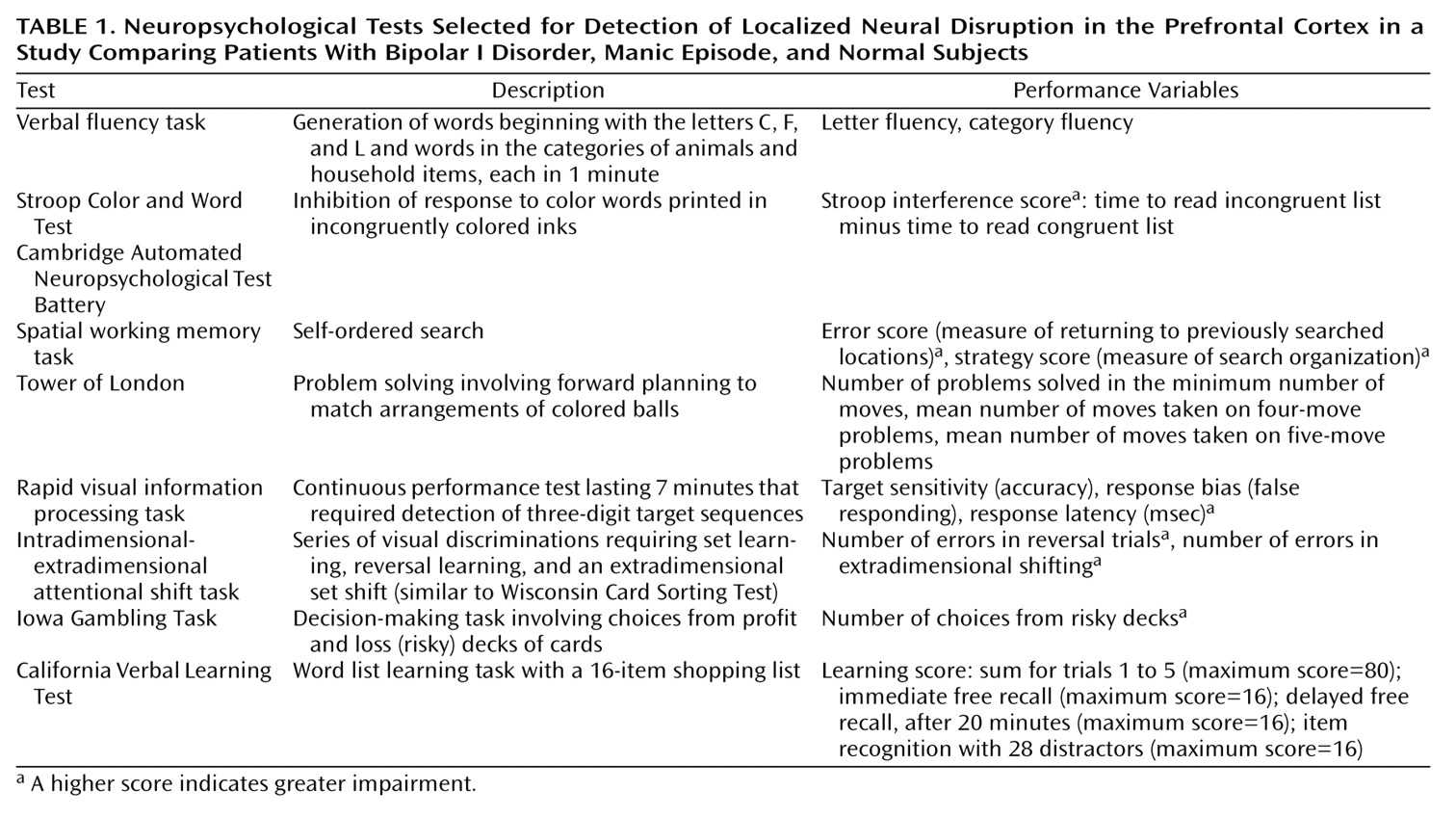
The renewed emphasis on identifying elemental psychological functions
(1) has led to the development of improved tests, especially tests of functions believed to be subserved by the prefrontal cortex, such as attention, working memory, planning, set learning, set shifting, and decision making. The effects of lesions provide a starting point for what we should expect of neuropsychological investigation in mood disorder. Lesions of the left frontal cortex have been reported to be associated with depressed mood, and lesions of the right frontal cortex with mood elevation
(2). A second emphasis has been on lesions of the inferior medial prefrontal cortex regardless of laterality, which appear to result in a disinhibition syndrome characterized by impulsivity, distractibility, and poor decision making
(3). These components of the frontal lobe syndrome are not clearly associated with disturbances of subjective or objective mood, but the overlap in apparent symptoms suggests that mania may have an inferior prefrontal component.
Specific neuropsychological investigations of patients with mania are just as limited at present. The existing findings suggest a pattern of generalized deficit across mnemonic and executive domains in mania
(6–
9). However, the clinical state of the manic groups has often been inadequately described, and studies have commonly relied on coarse tests that conflate several cognitive processes, such as the Wisconsin Card Sorting Test
(10,
11). In the neuropsychological study of mania reported here, we capitalized on the more recent development of tests for specific aspects of executive function by using items from the Cambridge Automated Neuropsychological Test Battery
(12) and the Iowa Gambling Test
(3). The latter test is believed to be specifically sensitive to damage in the ventromedial prefrontal cortex.
Results
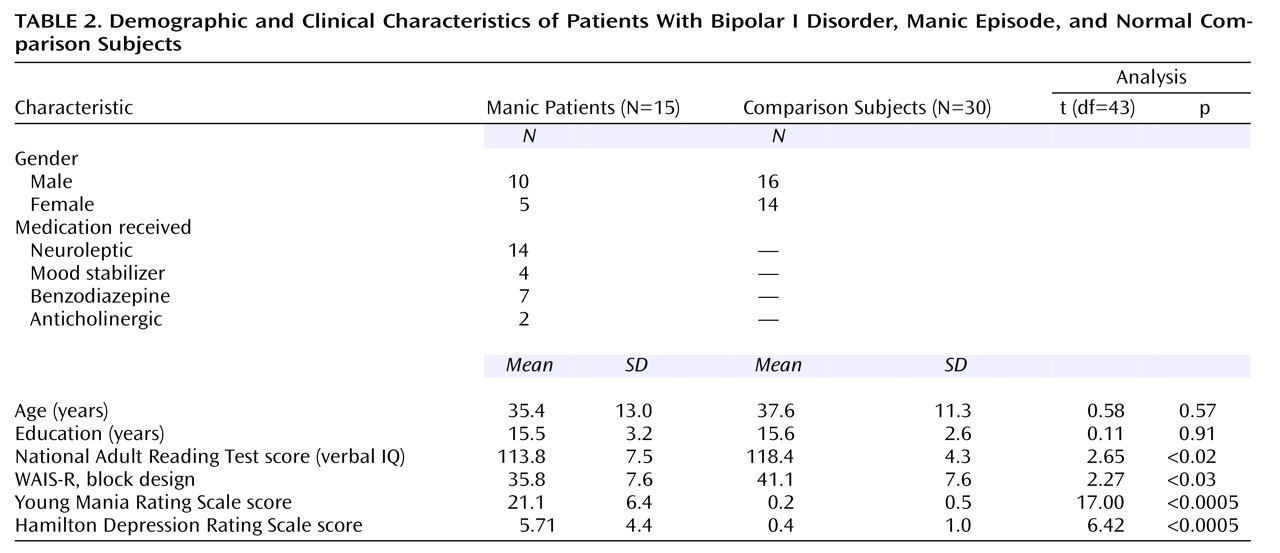
The demographic and clinical characteristics of the two groups are shown in
Table 2. The two groups were closely matched with respect to age and years of education; most had a university-level education. However, the patient group had significantly lower scores than the comparison group on the National Adult Reading Test and the WAIS-R block design test. These variables were included in the discriminant function analysis, but they failed to enter the model. All of the manic patients had ratings above 12 on the Young Mania Rating Scale (mean=21.1, SD=6.4). Only one patient was experiencing a first episode of mania. The other patients had a mean of 2.8 previous hospitalizations for manic illness (SD=2.6, range=0–9), and a mean length of time since initial diagnosis of 106.1 months (SD=117.8).
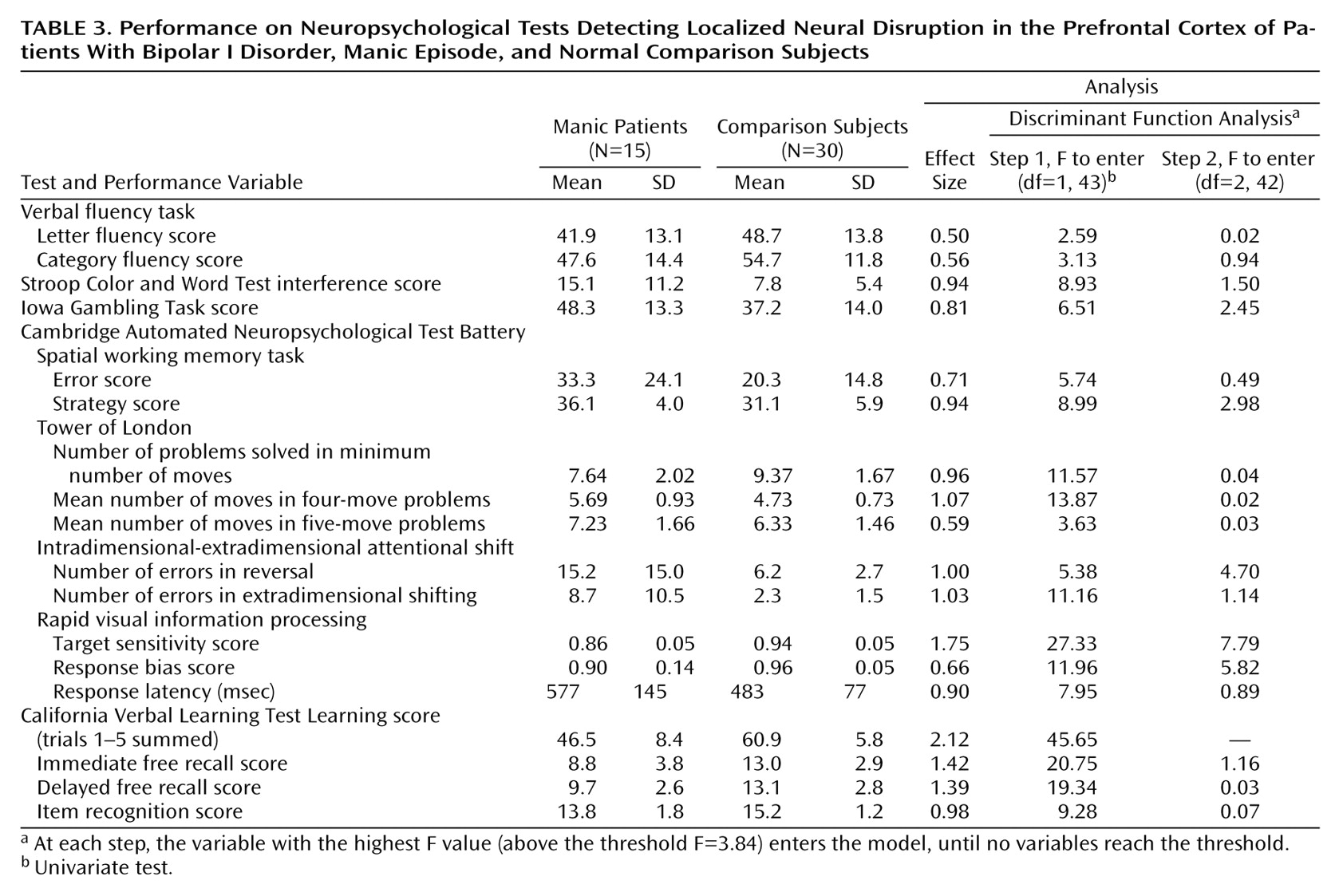
The subjects’ neuropsychological performance is summarized in
Table 3. The mean values indicate poorer performance in the manic study group on every dependent variable, although the effect sizes vary considerably. Missing data complicated the discriminant function analysis: three manic subjects refused to complete the spatial working memory task (the final test in the battery), and one manic subject refused to complete the Tower of London test (the penultimate test). To include these subjects and tests in the analysis, two separate analyses were performed with the missing values replaced in different ways: 1) by using the mean values for the two study groups combined (a more conservative method for detecting discrimination) and 2) by using the mean values for the manic study group (a less conservative method). Both analyses indicated the same discriminant function: only two variables reached inclusion criteria for the model. The F values presented in
Table 3 are from the second analysis. At step 1, the California Verbal Learning Test learning score entered (Wilks’s lambda=0.49, F=45.65, df=1, 43, p<0.00001), correctly classifying 84.4% of the subjects overall and 73.3% of the manic subjects. At step 2, the accuracy score for the rapid visual information processing task entered (Wilks’s lambda=0.41, F=7.79, df=2, 42, p<0.00001). The discriminant model correctly classified 91.1% of the subjects overall and 86.7% of the manic subjects. No additional variables met entry criteria at step 3.
A closer examination (post hoc) of results for the California Verbal Learning Test and the rapid visual information processing task revealed a significant interaction of learning trial (five levels, within subjects) and diagnosis (two levels, between subjects) on the California Verbal Learning Test (repeated measures analysis of variance [ANOVA]: F=5.51, df=4, 172, p=0.001) such that the manic subjects displayed less improvement with list repetition relative to comparison subjects. This finding suggests impairment in the encoding or consolidation of information into memory in the manic patients. For the rapid visual information processing task, which lasts 7 minutes, a repeated measures ANOVA revealed that the interaction of time (seven levels, within subjects) and diagnosis (two levels, between subjects) was not significant, i.e., the decrement in performance over time was no more pronounced in the manic subjects than in the comparison subjects.
Neuropsychological Performance and Medication
The effects of medication on performance in the manic group were analyzed in two ways. First, as 14 of 15 subjects were receiving neuroleptic medication, it was possible to examine correlations between neuroleptic dose (in chlorpromazine equivalents) and neuropsychological performance. Increasing neuroleptic dose was associated (albeit nonsignificantly) with improved performance on the rapid visual information processing task (r=0.36, df=13, p=0.19) but was not associated with California Verbal Learning Test performance (r=–0.04, df=13, p=0.96). Second, in light of the established effects of benzodiazepines and anticholinergic drugs on cognition
(20,
21), performance on the California Verbal Learning Test and the rapid visual information processing task were examined in a subgroup of manic patients who were free of these medications (N=6). For this subgroup, the mean California Verbal Learning Test learning score was 47.0 (SD=10.5) and the mean target sensitivity score for the rapid visual information processing task was 0.88 (SD=0.04). These values were very similar to the scores for the manic study group overall (compare with
Table 3). It is therefore unlikely that the deficits in verbal learning and sustained attention could be explained by medication status in the manic study group.
Neuropsychological Performance and Clinical Variables
Correlation coefficients were calculated for the association between neuropsychological performance and mood ratings on the Young and Hamilton mood rating scales for the manic patients. The score on the Young Mania Rating Scale correlated with impairment only on the intradimensional-extradimensional attentional shift task (r=0.64, df=13, p<0.01). No neuropsychological variables correlated significantly with either number of hospitalizations or duration of illness (all p>0.10).
Discussion
The manic phase of bipolar disorder is characterized by impaired performance across a range of tasks assessing executive, mnemonic, and attentional functioning. In the present study, a discriminant function analysis demonstrated that two measures, verbal learning (the California Verbal Learning Test) and sustained attention (the rapid visual information processing task), were particularly powerful indicators of the deficit in mania, correctly classifying 87% of manic subjects and 91% of subjects overall. The results of comparisons between the manic patients and the healthy subjects on the remaining five tests had effect sizes in the range of 0.5–1.0, representing moderate to large effects, but these measures did not significantly contribute to the classification of the manic subjects, suggesting that they do not represent core markers of the manic state.
A central hypothesis of our investigation was that the performance on the Iowa Gambling Task of the manic subjects would resemble that of patients with lesions to the ventromedial prefrontal cortex
(3). Although the manic subjects on average selected more cards from the risky decks than did the comparison subjects, they nonetheless chose more cards from the safe decks than from the risky decks overall and therefore did not closely resemble the patients with ventromedial prefrontal cortex lesions, who virtually ignore the safe decks. The differences between the manic subjects and the comparison subjects on the Iowa Gambling Task may be more readily explainable in terms of delayed acquisition, quite feasibly as a result of poor sustained attention or learning. Lesions to ventral aspects of the prefrontal cortex are also associated with impaired reversal learning
(22), an aspect of attentional processing assessed with the intradimensional-extradimensional attentional shift task. The manic subjects were impaired at several stages of this task, and it is suggested that this profile may be better accounted for by distractibility and impaired set learning than by a specific deficit in reversal learning. In conclusion, we were unable to demonstrate that the ventral prefrontal cortex was a major locus of dysfunction in acute mania.
Lateralized dysfunction of the prefrontal cortex has frequently been proposed as an underlying process in pathological affective states. The present data on sustained attention are consistent with a right prefrontal cortex abnormality in the manic state. Functional neuroimaging studies with the Continuous Performance Test, including the rapid visual information processing task, have consistently revealed a right-lateralized frontoparietal network of activation
(23,
24), and evidence from human subjects with lesions in this area corroborates the view that right frontal but not left frontal lesions disrupt sustained attention
(25). Furthermore, the task that showed the smallest effect size in the present study was verbal fluency, which has typically been associated with a
left-lateralized prefrontal locus
(17,
26). Other diverse lines of evidence support right prefrontal involvement in mania. Secondary mania after brain injury is more commonly associated with right frontal lesions than with left frontal lesions
(2). A preliminary study employing transcranial magnetic stimulation has reported that right but not left frontal transcranial magnetic stimulation may relieve symptoms of mania
(27). Finally, right-sided brain lesions in rats may have preferential effects on noradrenergic and dopaminergic neurotransmission, selectively resulting in behavioral overactivity
(28) and attenuated physiological activation to stress
(29).
It is less likely, however, that a core impairment in verbal learning in the manic state is also compatible with a right-lateralized prefrontal abnormality. The manic subjects showed pronounced impairment at the retrieval stages on the California Verbal Learning Test, but they also showed encoding difficulties. Their rate of learning was reduced, and their recognition ability was deficient. Encoding impairments on memory tasks are often interpreted in terms of medial temporal lobe dysfunction, while retrieval deficits may localize more frontally
(30). Altered temporal cortex metabolism has been reported in functional neuroimaging studies in mania, consistent with this account
(31,
32). However, imaging studies of mnemonic processing have highlighted contributions of the prefrontal cortex to both encoding (left-lateralized) and retrieval (right-lateralized)
(33). Furthermore, in light of the verbal nature of the California Verbal Learning Test, difficulties on the test suggest disruption of left hemisphere processes. The neural substrates of the mnemonic impairment in acute mania therefore require further elucidation, potentially through functional neuroimaging.
The manic study group was small, because recruiting and testing, as always, was difficult. The majority of previous studies had patient group sizes in the range of 14–20 subjects
(6–
10,
34). The manic subjects in the present study constituted a severely symptomatic group, with an average Young Mania Rating Scale score above 20, and suffered from few depressive symptoms. These data therefore reflect the effects of pure mania. Others have provided preliminary evidence of subtle neuropsychological differences between patients with mixed and pure mania
(35). In our study, 15 manic subjects completed the research. An additional nine subjects were fully cooperative, but meaningful data could not be obtained from them. It is almost an additional defining feature of severe acute mania that patients cannot be engaged in formal testing.
Sustained attention and mnemonic impairments have been noted in previous studies in manic groups. Sax and colleagues
(34) have reported a correlation in manic patients between sustained attention deficit and prefrontal and hippocampal volumes measured by magnetic resonance imaging. This correlation raises the interesting possibility that trait dysfunction in sustained attention may exist in bipolar disorder, as structural changes should be temporally stable. Preliminary data from our laboratory that have resulted from testing euthymic patients with bipolar disorder have indicated this is the case. Indeed, of the tasks employed in the present investigation, only sustained attention, measured with the rapid visual information processing task, remained impaired during the euthymic phase of bipolar disorder, when the analysis controlled for low levels of affective symptoms
(36). The trait nature of the attentional abnormality does not lend itself to a simple interpretation, since the postulated changes in monoamine function in mania might be expected to modulate attention as amphetamine does in normal subjects
(37).
Memory impairments have also been described in earlier reports in acute mania
(6,
8,
9), and verbal memory impairments have been robustly described in schizophrenia and unipolar depression
(38,
39). In contrast to the clear relevance of sustained attention to distractibility in mania, the relationship between memory impairment and the symptoms of mania is not immediately obvious. The reversibility of memory impairment in the euthymic state indicates that it is a state-related marker and implies a distinct neural substrate from that supporting sustained attention.
Significant impairments in manic groups relative to healthy comparison subjects on executive measures such as the Tower of London task
(8,
9), the Stroop Color and Word Test
(7), and the Cambridge Automated Neuropsychological Test Battery spatial working memory task
(9) have previously been reported. Although our findings support a weakness in executive processing in mania, executive tasks did not classify manic subjects as reliably as the California Verbal Learning Test and rapid visual information processing tasks. Consistent with this proposal, McGrath et al.
(7) reported significant impairment on a summary measure of executive (frontal) performance but results only approaching significance on three of the four individual tests (the Wisconsin Card Sorting Test, the Trail Making Test, and the verbal fluency task). The smaller effect sizes on executive tests in manic subjects may be explained in various ways, e.g., the prefrontal cortex may be only distantly related to the core pathology in mania, or it may be disrupted in only a subset of patients. The issue of “process purity” may also be important here: sustained attention and learning are component processes in many neuropsychological tests and thus deficits in these processes may lead to apparent generalized impairment.
In summary, deficits in verbal learning and sustained attention were capable of discriminating manic and normal comparison subjects better than measures of executive processing. A reduced capacity for sustained attention appears to be a trait abnormality in patients with bipolar I disorder and a clue perhaps to a key stable abnormality in the phenotype. Reduced episodic memory performance is very prominent in mania and depression, and its putative neurobiology is a neglected target for understanding of the state transitions in severe mood disorder.