Neuroleptic malignant syndrome is a rare but potentially fatal complication of neuroleptic drugs
(1). The syndrome is characterized by hyperpyrexia, extrapyramidal symptoms, catatonic symptoms, and autonomic disturbances. The pathophysiology of neuroleptic malignant syndrome has been explained mainly by a central hypodopaminergic state
(2–
4), although other neurotransmitters such as serotonin may be associated with it
(5).
There have been several reports on the familial occurrence of neuroleptic malignant syndrome
(2,
6,
7), suggesting that a genetic mechanism is involved in the predisposition to this syndrome. Therefore, we analyzed the coding sequences of the dopamine D
2 receptor gene (
DRD2) in 12 patients who had experienced neuroleptic malignant syndrome
(8). Although one patient had a rare heterozygote for the Pro310Ser variant in exon 7, the allele frequency was not significantly different in these patients from that in a control group.
The human
DRD2 gene contains a
TaqI restriction fragment length polymorphism, creating the A1 and A2 alleles
(9). Studies using postmortem brains showed that the subjects with one or two A1 alleles had lower DRD
2 density in the striatum
(10) and caudate nuclei
(11) than those without this allele, and these results have been confirmed in vivo with positron emission tomography
(12). Functionally, the A1 allele has been associated with diminished dopaminergic activity and reduced glucose metabolism in brain regions with abundant dopamine receptors
(11). These findings suggest the possibility that carriers of A1 show higher DRD
2 blockade by neuroleptic drugs and are more prone to develop neuroleptic malignant syndrome than noncarriers.
The goal of the present study was to examine the association between the TaqI A DRD2 polymorphism and the development of neuroleptic malignant syndrome.
Method
The subjects were 15 Japanese psychiatric patients who had developed neuroleptic malignant syndrome defined according to the criteria of Pope et al.
(13). Six of the patients were men and nine were women; their mean age was 42.5 years (SD=14.3). Twelve of the patients were suffering from schizophrenia and three from major depression diagnosed according to DSM-III-R. The comparison subjects were 138 Japanese patients with schizophrenia who had never developed neuroleptic malignant syndrome. Fifty-five of these patients were men and 83 were women; their mean age was 43.6 (SD=12.5).
The mean dose of neuroleptic in haloperidol equivalents and the duration of neuroleptic exposure were 10.4 mg/day (SD=6.7) and 127.7 days (SD=195.9), respectively, for the patients who had experienced neuroleptic malignant syndrome and 11.4 mg/day (SD=1.4) and 470.0 days (SD=645.4), respectively, for the comparison patients. This study was approved by the Ethics Committee of Hirosaki University Hospital. After complete description of the study to the subjects, written informed consent to participate was obtained from the patients and their families.
Ten ml of blood was obtained from each patient, and DNA was isolated from peripheral leukocytes by a guanidinium isothiocyanate method. The
TaqI A
DRD2 genotypes, the A1 and A2 alleles, were determined by the polymerase chain reaction method
(14).
The statistical analyses were performed with chi-square test with Yates’s correction or Fisher’s exact test (if the number of subjects in the cell was smaller than five) for categorical variables (e.g., gender distribution, allele frequency, and genotype distribution) and Student’s t test for continuous variables (e.g., age and neuroleptic dose). A two-tailed p value of 0.05 or less was regarded as statistically significant. All analyses were performed by using SPSS 8.0.1J for Windows (SPSS Japan, Inc., Tokyo).
Results
There were no significant differences in age (t=0.34, df=151, p=0.74), gender distribution (χ2=0.000, df=1, p=1.00), and neuroleptic dose (t=0.63, df=14.129, p=0.54) between the neuroleptic malignant syndrome patients and the comparison patients.
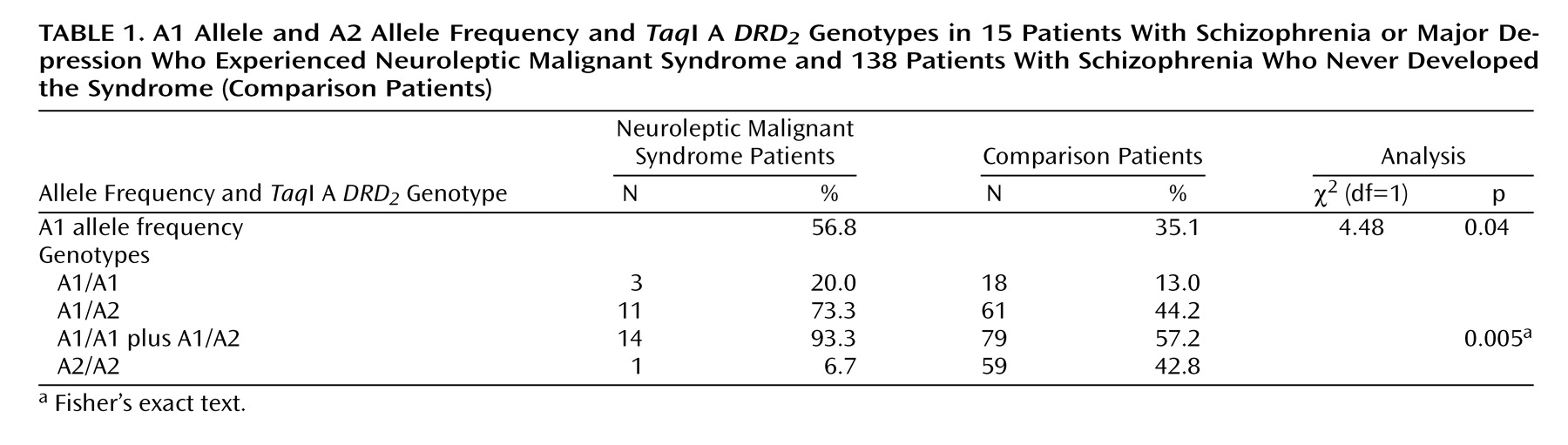
The A1 allele frequency was significantly higher in the neuroleptic malignant syndrome patients than in the comparison patients (
Table 1). The proportion of the A1 carrier was significantly higher in the neuroleptic malignant syndrome group than in the comparison group (
Table 1). The odds ratio of neuroleptic malignant syndrome occurrence in the A1 carriers compared with the noncarriers was 10.5 (95% confidence interval=1.34– 81.76).
Discussion
Several studies
(2–
4) have suggested that a central hypodopaminergic state is a basic part of the underlying pathophysiology in neuroleptic malignant syndrome. More specifically, Jauss et al.
(15), using single photon emission computed tomography, found a close association between the extent of DRD
2 blockade by neuroleptic drugs and the development of neuroleptic malignant syndrome. In a patient with schizophrenia, the DRD
2 blockade in the basal ganglia was complete during a neuroleptic malignant syndrome episode but unremarkable when the patient was free from the syndrome.
We recently reported that patients with schizophrenia who had the A1 allele showed greater prolactin response
(16) and better therapeutic response
(17) to nemonapride, a selective dopamine antagonist, than patients without this allele. These findings indicate that A1 carriers show higher DRD
2 blockade by neuroleptic drugs than noncarriers.
In the present study, the A1 allele frequency was significantly higher in the patients who had experienced neuroleptic malignant syndrome than in patients who had not had the syndrome. Surprisingly, 93.3% of the neuroleptic malignant syndrome patients carried one or two A1 alleles, and this proportion was significantly higher than it was among the comparison patients. The risk of developing neuroleptic malignant syndrome was estimated to be 10.5 times higher in the A1 carriers than in the noncarriers. These results suggest that the A1 allele is associated with the predisposition to neuroleptic malignant syndrome. It is likely that because of lower DRD
2 density
(10–
12), the A1 carriers have a higher chance than the noncarriers of showing excessive DRD
2 blockade by neuroleptic drugs, leading to the development of neuroleptic malignant syndrome.
It is still unclear how the
TaqI A polymorphism, which is located 10 kb into the 3′ untranslated region of the
DRD2 gene, affects the expression of this gene
(9). Most probably, this polymorphism reflects linkage disequilibrium with some unidentified mutations that alter DRD
2 density
(9). Therefore, in the event of clarification of those gene mutations, their relationships with the development of neuroleptic malignant syndrome should be examined.
A possible clinical implication of our findings are that A1 carriers could receive potentially lower doses of neuroleptics if this polymorphism were used in a pharmacogenomic screening procedure. To confirm these findings, further replicated studies should be performed with larger numbers of subjects.