Anxiety disorders are among the most common diagnoses reported in childhood and adolescent epidemiological studies
(1–
3). Costello
(4) found that 8.9% of a pediatric sample met criteria for an anxiety disorder, and Kashani and Orvaschel
(5) reported prevalence rates of 17.3% for any anxiety disorder in a group of adolescent subjects. The reported prevalence rates for the childhood disorders are based on the DSM-III and DSM-III-R diagnosis of overanxious disorder, which was incorporated into the category of generalized anxiety disorder in DSM-IV. In community epidemiological studies, the prevalence rates for overanxious disorder have ranged from 2.9% to 4.6%, and rates for separation anxiety disorder have ranged from 2.4% to 4.1%
(6–
8). Kashani and Orvaschel
(5) reported prevalence rates of 7.3% for overanxious disorder and 7.0% for separation anxiety disorder in adolescent subjects. Kendall and Warman
(9) made diagnoses with the DSM-IV criteria for generalized anxiety disorder in a large group of children with overanxious disorder and found that the majority of children with overanxious disorder met the criteria for generalized anxiety disorder. Therefore, the prevalence rates reported for overanxious disorder provide an approximation of the prevalence of generalized anxiety disorder in children.
In addition, more general social withdrawal (evidenced by anxiety, isolation, hypersensitivity, depression, and self-consciousness) that interferes with functioning has been reported in 10%–20% of school-aged children
(10,
11). Children with anxiety disorders often struggle with low self-esteem, social isolation and inadequate social skills, academic difficulties
(12,
13), and physical problems such as headaches, stomachaches, and irritable bowel syndrome
(14).
There are limited data on the psychopharmacological treatment of anxiety disorders, with the exception of obsessive-compulsive disorder
(15), even though the use of psychotropic medication has increased dramatically in the last few years
(16). There are few adequately designed psychopharmacological studies to guide treatment of childhood generalized anxiety disorder
(17,
18). Only a few studies, with small numbers of subjects, have examined the use of benzodiazepines in the treatment of childhood anxiety disorders
(19,
20). However, the potential risk of sedation, dependence, and withdrawal associated with the benzodiazepines effectively rule these medications out as a front-line treatment. Four double-blind, placebo-controlled studies have examined the use of tricyclic antidepressants for patients whose main complaints were school refusal associated with separation anxiety disorder
(21–
24). These studies provided contrasting results, and only one found significant positive effects. On additional study recently reported that the combination of imipramine and cognitive behavior therapy was significantly more effective than cognitive behavior therapy alone in the treatment of school refusal in adolescents with comorbid depression and anxiety
(25).
The safety of the selective serotonin reuptake inhibitors (SSRIs) recommends them as potential candidates for the treatment of childhood generalized anxiety disorder and separation anxiety disorder, as does SSRIs’ notable effectiveness in treating depression, which is commonly comorbid with childhood generalized anxiety disorder and separation anxiety disorder
(26). The clinical similarity between generalized anxiety disorder and separation anxiety disorder and the favorable response of adult patients with generalized anxiety disorder to SSRIs such as paroxetine
(27) and venlafaxine
(28,
29) provide a rationale for testing these medications in younger populations. Fluoxetine has shown some preliminary benefit in small pilot case series in children and adolescents with anxiety disorders
(30–
32), and fluvoxamine has recently been shown to benefit children and adolescents with a variety of anxiety disorder diagnoses
(33).
To our knowledge, the current study is the only randomized, double-blind, placebo-controlled trial of an SSRI (sertraline) in children and adolescents with generalized anxiety disorder as the primary diagnosis. The children and adolescents in the study were carefully assessed with a structured clinical interview, and their symptoms and side effects were monitored at each visit by using a structured protocol. Outcome was multimodal and included both clinicians’ ratings and self-reports of anxious and depressive symptoms.
Method
Sertraline was chosen as a representative of the SSRI class of antidepressants. Children and adolescents with a primary diagnosis of generalized anxiety disorder underwent a 2–3-week prestudy evaluation period, followed by a 9-week double-blind treatment phase in which they received either sertraline or placebo.
The medication and placebo were prepared in identically appearing capsules, and random study group assignments were made in groups of four subjects, with each group receiving two placebo and two sertraline treatment assignments. Sertraline capsules contained 25 mg for the first week and 50 mg for weeks 2–9. The study medication was prescribed to be taken once daily in the evening. The patients were seen weekly. Each week they received a bottle with 10 capsules to allow for a few extra days in case of a problem in scheduling the next visit. Compliance with the medication regimen was assessed by pill count. All patients tolerated 50 mg of sertraline well; therefore, no dosage adjustment was necessary during the study.
A trained research coordinator performed a brief telephone screen interview to determine the child’s study eligibility. Children who appeared to meet generalized anxiety disorder criteria and did not meet any of the exclusion criteria were scheduled for an initial screening evaluation. Families were then sent a variety of assessment measures, including symptom ratings and background information questionnaires, and were instructed to complete them and bring them to the intake visit.
Patients’ eligibility for the study was determined during a screening evaluation over two visits. These visits included a structured clinical interview and completion of the Anxiety Disorders Interview Schedule for Children—Revised
(34), administered by the authors (M.A.R., a board-certified child and adolescent psychiatrist, and L.S., a child psychologist). All patients who met the study intake criteria were offered participation. The details of the study design and treatments were explained, participants’ questions were answered, and written informed consent obtained from the parent and from children ≥12 years of age and assent from children <12 years of age. The study was approved by the Institutional Review Board at the University of Pennsylvania. On either the first or second screening visit and before entering the randomized treatment phase, all children and adolescents received a physical examination, including an ECG and laboratory tests. Patients who still met study intake criteria at the end of the screening period returned 1 week later for a baseline evaluation, where the results of the Anxiety Disorders Interview Schedule for Children—Revised were reviewed with the parent and the patient. Patients who continued to meet study criteria at this visit were entered into the double-blind study and received either sertraline or placebo. During the study, children and adolescents were encouraged not to consume alcohol and not to take over-the-counter medications without first consulting the study physician.
The nine weekly medication management visits with the authors (M.A.R. or L.S.) ranged from 20 to 30 minutes in length. These visits included a check on medication compliance, a review of medication side effects, and an assessment of symptoms and global clinical status. Structured psychotherapy was not offered, but support and encouragement were provided. Weight, pulse, and blood pressure were assessed at each visit. Patients were required to undergo a second safety evaluation, including a physical examination, ECG, and laboratory tests, at the end of the study. At the conclusion of the study, patients were offered treatment with sertraline. Those who wished to discontinue the medication had their dose tapered over 1 week. Patients were given the option of receiving up to 3 months of free follow-up visits before being referred for community treatment.
Subjects
Patients ranging in age from 5 to 17 years were drawn from referrals by psychiatrists and pediatricians. The study was conducted in the Child and Adolescent Research Service in the Mood and Anxiety Disorders Section of the Department of Psychiatry, University of Pennsylvania. To be eligible for the study, patients had to meet DSM-IV criteria for generalized anxiety disorder, according to the Anxiety Disorders Interview Schedule for Children—Revised, and have a Hamilton Anxiety Rating Scale
(35) score ≥16. At least one primary caretaker or parent had to be willing to participate in study evaluations and accompany the patient at all visits. Finally, psychotherapy, except for cognitive behavioral therapy, was allowed, providing patients had been receiving the same therapy for the past 3 months and the level of intensity was unchanged throughout the study. Patients were excluded from the study if they suffered from acute or unstable medical conditions such as diabetes, seizure disorder, severe asthma, or hyperthyroidism. Patients were also excluded if they had an additional axis I or axis II psychiatric disorder, such as major depressive disorder, obsessive-compulsive disorder, mental retardation, pervasive development disorder, eating disorder, schizophrenia, or other psychotic disorders. Children with comorbid attention deficit hyperactivity disorder and oppositional defiant disorder were excluded, given the small size of the study group and our interest in testing medication treatment in a group of patients with anxiety only. Finally, patients who were at risk for suicide and/or who had abnormal results on the physical examination or laboratory tests were excluded. However, six patients who had subsyndromal symptoms of separation anxiety disorder were accepted because generalized anxiety disorder was their primary diagnosis. Given the small size of the study group, the exclusion criteria were rather strict.
Assessments
Eligibility criteria were assessed with the Anxiety Disorders Interview Schedule for Children—Revised, administered by the clinician separately to both child and parent. The Anxiety Disorders Interview Schedule for Children—Revised asks both the child and the parent to rate the severity of anxiety in terms of level of distress and impairment of functioning on a scale from 1 to 8. Primary outcome measures were the Hamilton anxiety scale
(35) total score and psychic and somatic factor scores and the Clinical Global Impression (CGI) severity and improvement scale scores
(36). Assessments were completed weekly. Secondary assessment instruments included several factors of the Multidimensional Anxiety Scale for Children
(37,
38), the Revised Children’s Manifest Anxiety Scale
(39), and the 17-item Hamilton Depression Rating Scale
(40). These scales were completed at baseline and after 4 and 9 weeks of treatment. In addition, a side effect inventory
(41) was used to assess the presence or absence of side effects. The clinician reviewed all symptoms endorsed by the child or the parent and provided a severity rating after meeting with the child and parent.
Statistical analysis
Differences in demographic and clinical variables at baseline between the sertraline group and the placebo group were tested with t tests for continuous variables and Fisher’s exact test for categorical variables. Differences in adverse events were evaluated with Fisher’s exact test. The primary continuous outcome measures were evaluated by using a repeated measures analysis of covariance (ANCOVA) mixed model with an unstructured covariance matrix that incorporated correlations for all of the observations arising from the same subject
(42). The model had treatment (sertraline versus placebo) as a fixed factor and time (outcome scores at weeks 1, 2, 4, 6, 8, and 9) as the repeated factor and contained an interaction (treatment-by-time) term to evaluate the difference in slopes between treatments. The outcome measure at baseline was used as covariate. Any significant findings were followed up by individual ANCOVAs conducted for all time periods. Analyses of the secondary outcome measures were performed with ANCOVA for scores at week 9, with the last observation carried forward and baseline scores as covariates. Global improvement after 9 weeks of treatment was assessed with Fisher’s exact test. All tests were two-tailed and had an alpha level of 0.05.
Results
Patients’ Demographic and Clinical Characteristics
Thirty-seven patients met diagnostic criteria for generalized anxiety disorder and began the screening period. At the completion of the two screening visits, seven patients had improved and no longer met inclusion criteria. Another eight continued to meet inclusion criteria but for various reasons declined participation. The 22 remaining patients, who ranged in age from 5 to 17 years, were randomly assigned to treatment groups. No differences were found between the patients who were assigned to treatment groups (N=22) and those who were excluded (N=15) in mean age (mean=11.7, SD=3.9, and mean=10.9, SD=3.4, respectively; t=0.64, df=35, p<0.53), proportion of white subjects (81% and 92% respectively; χ2=0.82, df=1, p<0.36), or proportion of male subjects (77.3% and 57.1%, respectively; χ2=1.63, df=1, p<0.20). Seventy percent of the subjects’ parents were married, and the majority of parents had a college education (63% of mothers and 47% of fathers) (data on seven fathers were missing). More than 90% of the fathers and 73% of the mothers were employed. The Hamilton anxiety scale total score at baseline ranged from 16 to 27 (placebo group: mean=23.3, SD=4.0; sertraline group: mean=20.6, SD=3.6; t=1.69, df=20, p<0.11). The mean CGI severity scale score was 4. The duration of illness for all but one patient was more than 12 months. Three patients were previously treated with methylphenidate, one with paroxetine, and one with alprazolam. Three patients saw a school counselor regularly, and six patients were in private psychotherapy before enrollment in the study. Two patients suffered from asthma, two from seasonal allergies, and one from migraine headaches. One patient complained of gastric distress. No other medical conditions were present.
Attrition
Of the 22 patients randomly assigned to the treatment groups, only two patients in the placebo group and one patient in the sertraline group dropped out before completing the study, one each after 1, 2, and 4 weeks of treatment. All three patients were unimproved. This total attrition rate of 14% is rather low for a 9-week clinical trial.
Adverse Events
Adverse events were assessed with a checklist of items, each of which were rated on a scale from “not present” to “mildly,” “moderately,” and “markedly present.” A symptom was defined as an adverse event either if it was not present at baseline but was reported in the course of the study or if it was present at baseline but its severity was increased by at least 1 point on the scale during the study. Fisher’s exact tests (p<0.05) showed no statistically significant differences in adverse events between the sertraline group and the placebo group. It is interesting to note that patients who received sertraline reported less dizziness (18% versus 64%, p<0.08), nausea (5% versus 55%, p<0.06) and stomach pain (18% versus 64%, p<0.08) (Fisher’s exact tests) than the patients who received placebo. Only for four side effects did the sertraline patients report numerically more adverse events than the placebo patients: dry mouth (55% versus 27%, p=0.39), drowsiness (73% versus 45%, p=0.39), leg spasms (36% versus 9%, p=0.31), and restlessness (55% versus 27%, p=0.39) (Fisher’s exact tests).
Clinical Improvement
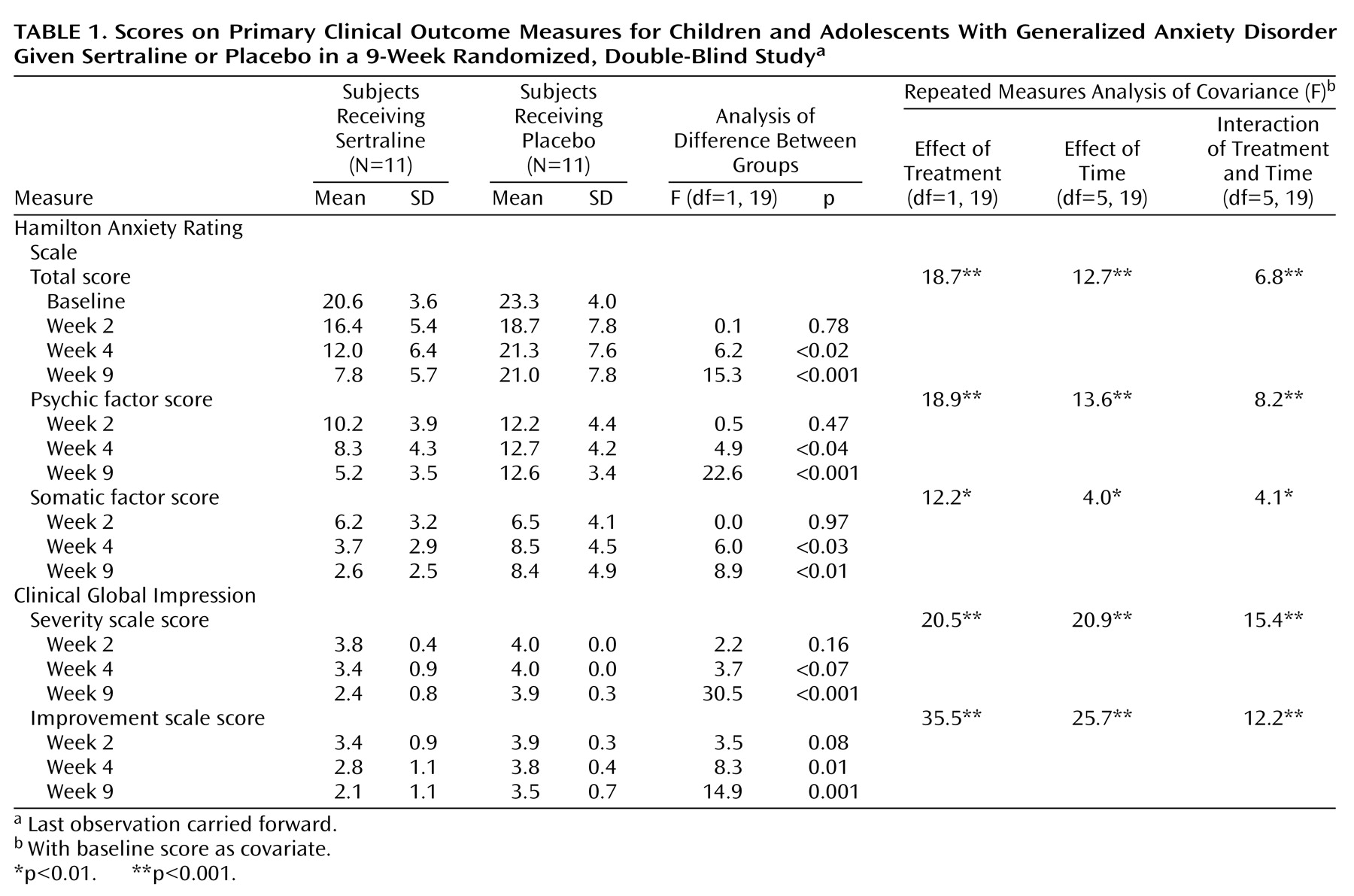
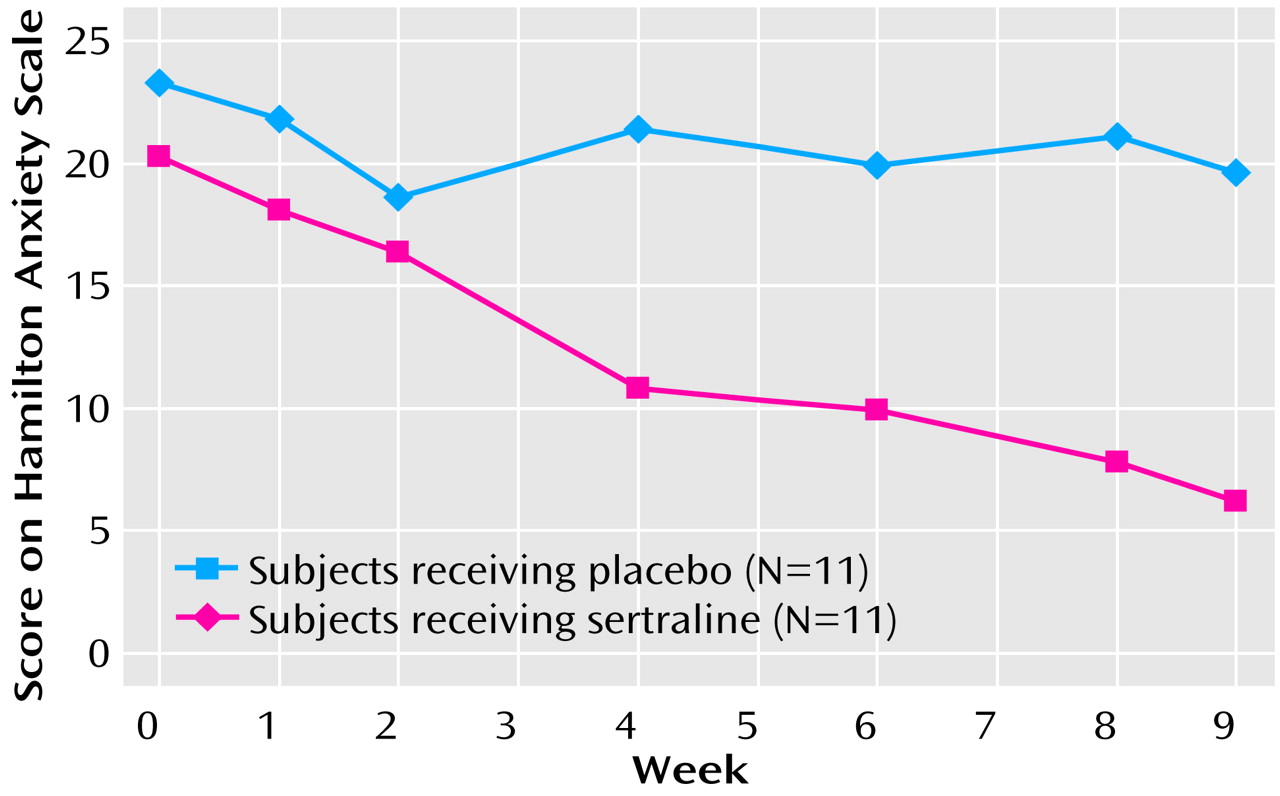
The primary measures, the Hamilton anxiety scale total score and psychic and somatic factor scores and the CGI severity and improvement scale scores, showed significant treatment differences in favor of sertraline from week 4 to the end of the study (
Table 1). Highly significant treatment effects were observed for all five outcome variables. Data for the Hamilton anxiety scale total score are presented graphically in
Figure 1.
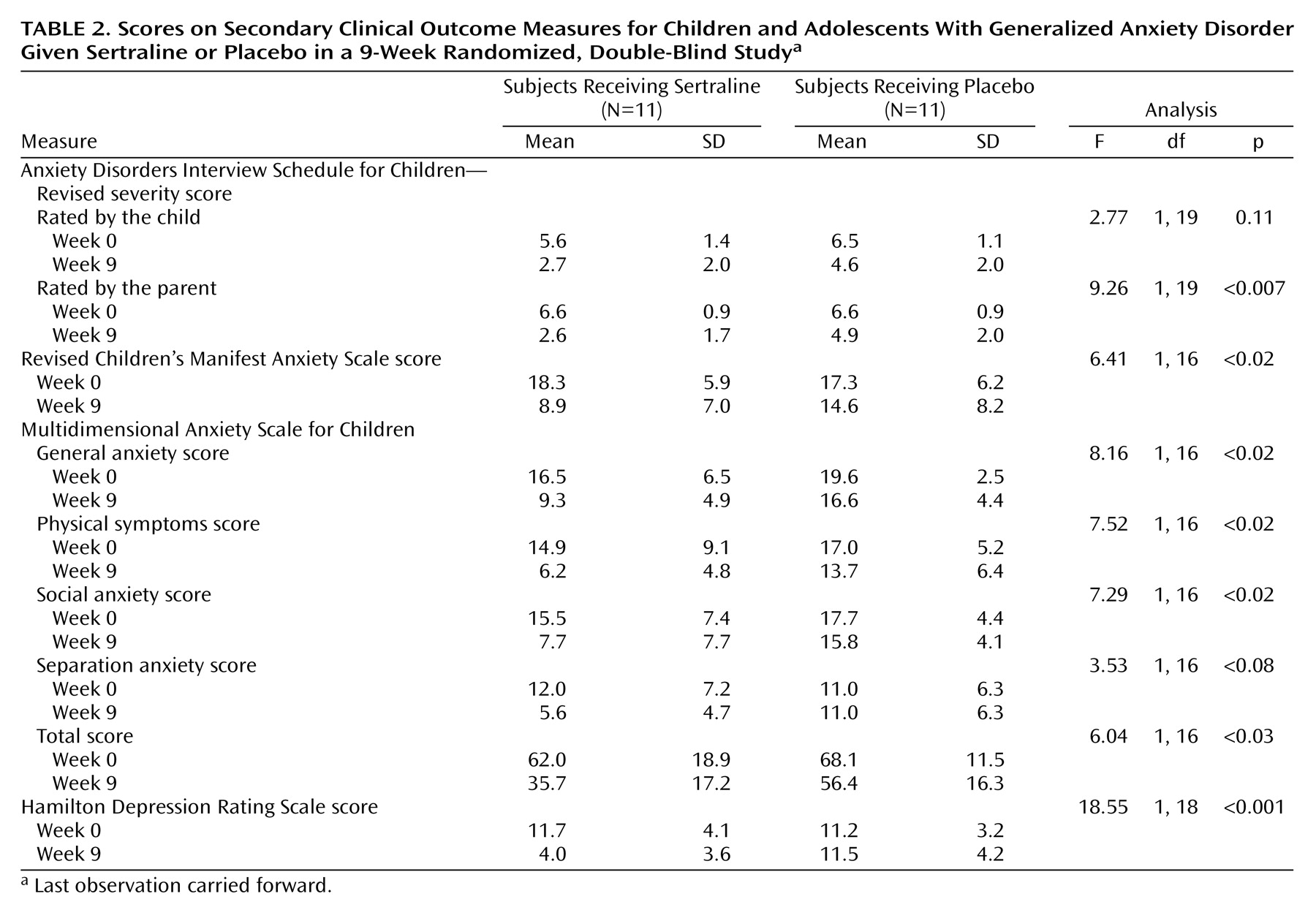
Table 2 reports 9-week outcome results for the secondary outcome variables, the Anxiety Disorders Interview Schedule for Children—Revised severity score rated by both the child and parent, the Revised Children’s Manifest Anxiety Scale completed by the child, several factors of the Multidimensional Anxiety Scale for Children, and the Hamilton depression scale. Significant differences in favor of sertraline were observed for the Hamilton depression scale, the Anxiety Disorders Interview Schedule for Children—Revised severity rating by the parent, the Revised Children’s Manifest Anxiety Scale score, and the Multidimensional Anxiety Scale for Children total score and generalized anxiety, physical symptom, and social anxiety factor scores. No statistically significant differences were found in the Anxiety Disorders Interview Schedule for Children—Revised severity rating by the child and Multidimensional Anxiety Scale for Children separation anxiety factor score.
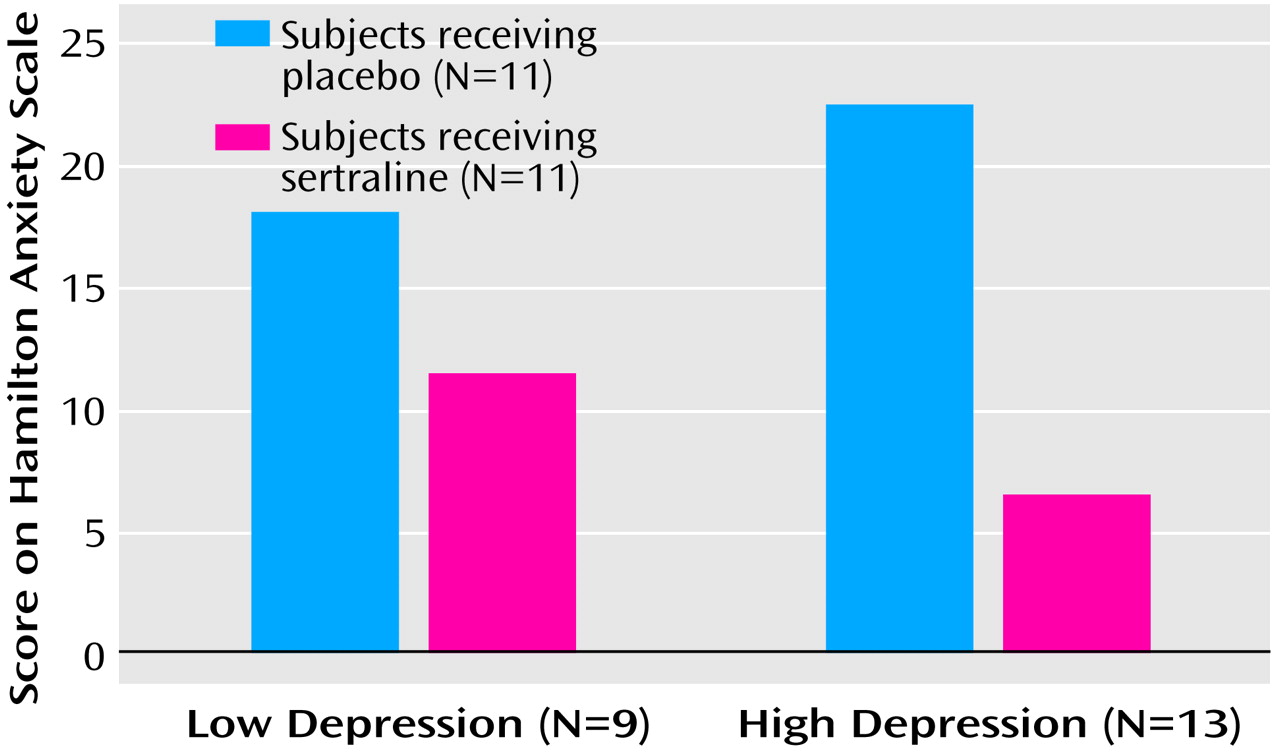
To assure that the anxiolytic effects of sertraline were independent of its antidepressant effects, we conducted an additional analysis of outcomes in groups of patients with low Hamilton depression scale scores (≤10) (N=9) and high scores (>10) (N=13). Conducting a factorial analysis of covariance with adjustment for baseline score on the Hamilton anxiety scale, we again obtained a highly significant main treatment effect (F=11.0, df=1, 17, p<0.005) in favor of sertraline, but no main depression severity effect (F<0.1, df=1, 17, p=0.92) and no depression-by-treatment interaction effect (F=2.5, df=1, 17, p=0.13) (
Figure 2). To ensure that the results were independent of age effects, we compared Hamilton anxiety scale scores from baseline to week 9 for patients age <12 years (N=11) and those age ≥12 years (N=11) (mean=–8.0, SD=8.5, versus mean=–7.0, SD=9.2, respectively; (t=0.27, df=20, p<0.79). No age effects on treatment outcome were found. Considering patients rated as moderately or markedly improved (CGI improvement scale scores=1 or 2) as “improved,” and those rated as minimally improved or unimproved (CGI improvement scale scores=3 or 4) as “unimproved,” we observed that at treatment endpoint, 10 of the 11 patients who received sertraline (90%), but only one of the 11 who received placebo (10%), were improved (p<0.001, Fisher’s exact test). However, of those patients who improved while taking sertraline, only two patients were markedly improved, representing a possible remission rate of only 18%.
Discussion
The results of this double-blind, placebo-controlled trial suggest that the SSRI sertraline is safe and efficacious for the symptomatic treatment of generalized anxiety disorder in children and adolescents at the low daily dose of 50 mg. Psychiatrist- and patient-completed rating scales showed significantly more symptom reduction in patients who received sertraline than in those who received placebo. There were no differences between the placebo and sertraline groups in side effects reported. On the basis of experience gained with adults
(28,
43), we speculated that medication might have the most impact on psychic and not somatic symptoms of anxiety. In this study, however, sertraline caused significant reduction in both psychic and somatic symptoms of anxiety.
Some comments about diagnosing children with anxiety disorders according to DSM-IV criteria appear appropriate. DSM-IV includes overanxious disorder in the category of generalized anxiety disorder and avoidant disorder in the category of social phobia. Although generalized anxiety disorder in adults is characterized by excessive anxiety and worry that the person finds difficult to control in a variety of areas, less evidence is available on how generalized anxiety disorder manifests itself in children and adolescents, as only a few studies that have used the DSM-IV criteria have been published. However, generalized anxiety disorder and overanxious disorder share many features, and one study found that most children with overanxious disorder also met criteria for generalized anxiety disorder
(9).
Our research and clinical experience suggest that there may be limitations in the definition of generalized anxiety disorder in children and adolescents. In this study, children and adolescents were diagnosed by using a structured diagnostic interview conducted with both the parent and the child, and the study subjects were required to have a primary diagnosis of generalized anxiety disorder. All of the children indeed reported worry in multiple areas, but some also had separation concerns with primary attachment figures, and almost all of them had some social anxiety. Some children in fact had multiple fears, significant impairment, and avoidance of important developmental tasks (missing school and lacking social involvement outside the family).
Limitations of the study included the small size of the study group. Replication of study results in a larger group is necessary. In addition, most but not all assessments were completed by one of the authors (M.A.R.), although she was blind to the treatment condition. The lack of difference in the side effect profile between the placebo and sertraline groups suggests that the double blind was maintained. Finally, in terms of measurement, the number of clinician- and patient-rated scales for anxiety disorders in children and adolescents is limited. We used the adult Hamilton anxiety scale successfully, yet formal validity and reliability data for use of this instrument with children are not available. The minimal placebo response found in this study is noteworthy. Because of the small size of the study group, it is difficult to speculate about its causes. However, the parents’ reports of change were equally considered when rating the CGI. Finally, the children included in this study had struggled with the symptoms of generalized anxiety disorder for several years. It may be that the physical symptoms and behavioral manifestations of anxiety symptoms are less amenable to a placebo response than more experiential symptoms of level of distress.
Future studies to examine minimum dose requirements, maximum duration of therapy, relapse rates after various treatment intervals, and a comparison of medication to cognitive behavior treatment are appropriate.