Social phobia is the third most common psychiatric disorder after major depression and alcohol abuse
(1). Individuals with generalized social phobia or social anxiety disorder fear and avoid performance and interactional situations where they may say or do something to embarrass or humiliate themselves. The experience of some degree of social anxiety is neither unusual nor pathological. Social phobia is distinguished by the intensity of the social anxiety and by the accompanying impairment or disability. Patients with generalized social phobia may have few friendships, experience trouble dating, drop out of school prematurely, reject promotions at work, become demoralized and depressed, abuse alcohol, and develop other comorbid psychiatric disorders
(2,
3). Epidemiologic reports place the point prevalence of generalized social phobia in the community at 4%–5%
(4,
5).
Treatments that have been shown to be efficacious for generalized social phobia include nonpharmacological interventions such as cognitive behavior therapy and social skills training
(6) and pharmacological treatments such as the irreversible and reversible monoamine oxidase inhibitors (MAOIs)
(7–
10) and high-potency benzodiazepines
(11). The reversible MAOI moclobemide has shown equivocal efficacy in several studies
(12,
13).
Short-term (12 weeks or less), placebo-controlled studies also support the efficacy of the selective serotonin reuptake inhibitors (SSRIs) for generalized social phobia
(14–
17). The large-scale, multicenter, double-blind, placebo-controlled study of sertraline for the medium-term treatment of generalized social phobia on which the current paper is based was designed to confirm and further characterize the findings of earlier open, single-center, placebo-controlled studies with sertraline
(14).
Method
We conducted a double-blind, placebo-controlled trial in patients with generalized social phobia, whose diagnosis was confirmed with the Structured Clinical Interview for DSM-IV—Patient Edition
(18). Investigators at 10 outpatient anxiety clinics in Canada participated in this 20-week study. Prestudy investigator meetings included training on rating scales and interrater reliability assessments on primary efficacy measures. The institutional review boards at all of the centers approved the protocol. Written informed consent was obtained after the study procedures had been fully explained to the patients.
Following initial screening procedures, potential subjects entered a 1-week, single-blind, placebo run-in period. Patients who continued to meet all inclusion criteria and did not have a Clinical Global Impression (CGI)
(19) severity score decline of 2 points or more were randomly assigned to receive sertraline or placebo in a ratio of 2:1. Following the 20-week treatment period, patients responding to sertraline were randomly assigned again to either continue sertraline or to switch to placebo for a further 24 weeks. The second phase of the study will be reported on separately. The endpoint of the current study was at the end of the first phase, 20 weeks after baseline.
Patients received an initial dose of 50 mg/day of sertraline or matching placebo. After 4 weeks, the dose could be increased by 50 mg/day every 3 weeks in the absence of satisfactory response (CGI improvement score indicating much or very much improved) up to a maximum allowable dose of 200 mg/day by week 10. The dose could be reduced to a minimum of 50 mg/day if required by the presence of intolerable side effects.
Urinary screening to detect occult benzodiazepine use was conducted at baseline and at week 2. Follow-up screening was performed at subsequent visits if necessary. The only sleep medications permitted during the study were chloral hydrate (500–1000 mg per night) or zopiclone (3.75–7.5 mg per night).
Patients
Subjects were recruited from newspaper advertisements, media reports, and clinician referrals. Inclusion criteria for the study required patients to meet DSM-IV criteria for primary generalized social phobia of at least 1-year duration at screening. Patients had to have a CGI severity rating of 4 or less (i.e., moderately ill or worse) and to be between 18 and 60 years of age without any serious or uncontrolled medical illness or condition that precluded sertraline use.
Patients with an additional diagnosis of avoidant personality were allowed to participate. Patients with comorbid major depression were permitted to enter the study provided their diagnosis was secondary to social phobia, their baseline Montgomery-Åsberg Depression Rating Scale
(20) score was 19 or less, the onset of social phobia predated onset of the current episode of depression by 5 years or more, and the patient did not represent a substantial suicide risk. Patients were excluded if they had another primary axis I disorder or fulfilled criteria in the previous 6 months for panic disorder, agoraphobia, obsessive-compulsive disorder, eating disorders, body dysmorphic disorder, or substance abuse.
Other exclusion criteria included concomitant use before study screening of psychotropic medication within a period of 5 half-lives, neuroleptics within 7 months, serotonergic antidepressants or an antianxiety medication for 3 or more weeks within 3 months, and cognitive behavior therapy within 4 weeks. Patients receiving benzodiazepines were permitted to enter the study after completing a minimum 2–4-week tapered discontinuation. Additional reasons for exclusion included a urinary screen positive for benzodiazepines at baseline, treatment with β-blockers or clonidine, and participation in a clinical trial within the previous 12 months. Women who were pregnant, lactating, or not using an acceptable method of contraception were excluded, as were patients who had had a major life event in the last 3 months that, in the investigators’ opinion, was influencing their current condition.
Study Procedures
Subjects were evaluated at weeks 1, 2, 4, 7, 10, 13, 16, and 20. The primary efficacy variables were 1) the percentage of responders at endpoint, defined as those rated on the CGI improvement item as 1 (very much improved) or 2 (much improved); 2) the mean change in total score from baseline to endpoint on the patient-rated five-item social phobia subscale of the Marks Fear Questionnaire
(21); and 3) the mean change in total score from baseline to endpoint on the Brief Social Phobia Scale
(22,
23), a physician-rated 11-question scale assessing fear and avoidance of and physiological response to performance and social situations.
Physician-rated secondary efficacy variables included the CGI severity and CGI improvement mean scores and the CGI overall severity of illness and CGI change measure of the Liebowitz Panic and Social Phobic Disorders Rating Form
(24). Patient-rated secondary efficacy variables included the Social Phobia and Anxiety Inventory social phobia subscale
(25), the Social Avoidance and Distress Scale
(26), and the Fear of Negative Evaluation Scale
(26).
Levels of depressive and general anxiety symptoms were assessed on the Montgomery-Åsberg Depression Rating Scale
(20) and the Clinical Anxiety Scale
(27), respectively. Quality of life was assessed with the Sheehan Disability Scale
(28,
29).
Safety assessments included evaluation, at each visit, of vital signs (weight, blood pressure, and heart rate) and the recording of spontaneously reported or observed adverse events. In addition, the use of concomitant medication was recorded, and compliance was monitored by pill counts of returned medication.
Statistical Procedures
Patients who had received at least one dose of double-blind medication and at least one postbaseline safety evaluation were defined as the safety evaluable group. Efficacy analyses were carried out on the intent-to-treat group, which was defined as subjects who had received at least one dose of double-blind medication and at least one postbaseline efficacy evaluation.
The number of patients calculated to be necessary to ensure 80% power at an alpha level of 0.05 (two-sided) to detect a 30% difference in CGI improvement response rates, a five-point difference in the Brief Social Phobia Scale total scores, or a four-point difference in the scores on the social phobia subscale of the Marks Fear Questionnaire with a 20% attrition rate was approximately 180 (120 patients receiving sertraline and 60 patients receiving placebo). For the primary efficacy measures, the between-treatment comparison at endpoint was of primary interest.
Chi-square tests and t tests were used to compare baseline characteristics of the patients receiving sertraline or placebo. The main efficacy analyses were performed by using repeated measures analysis of covariance (ANCOVA), with baseline measures as covariates. ANCOVA models included the effects of treatment, investigative site, and interaction of treatment and investigative site. The treatment-by-investigative site interaction terms were included in the final models although they were examined and found nonsignificant at alpha=0.1 for the main primary efficacy measures
(30). All statistical tests were two-sided and assumed a 0.05 level of significance except for tests of interactions, which were considered significant if p<0.1. Correlation analyses between baseline values and endpoint changes in the primary efficacy measures and changes in Montgomery-Åsberg Depression Rating Scale scores were performed.
The frequency of adverse events and the proportion of patients who left the study because of adverse events were compared between treatment groups with chi-square tests. Changes in vital signs were compared between treatment groups with ANCOVA.
The improvement slopes for patients in the sertraline and placebo groups were compared in random regression analyses. The mixed quadratic model over visit week in the random regression analyses included treatment group as the main effect, patient as the random effect, and baseline scores as the covariates. Random regression was used to compare improvement slopes because it makes fewer assumptions about missing data while optimizing the use of available data. Furthermore, random regression analysis allows comparison of improvement slopes where the unequal time interval of visits is taken into account; ANCOVA compared means by visit, without scaling differences in time between visits.
Results
Baseline Clinical and Demographic Characteristics
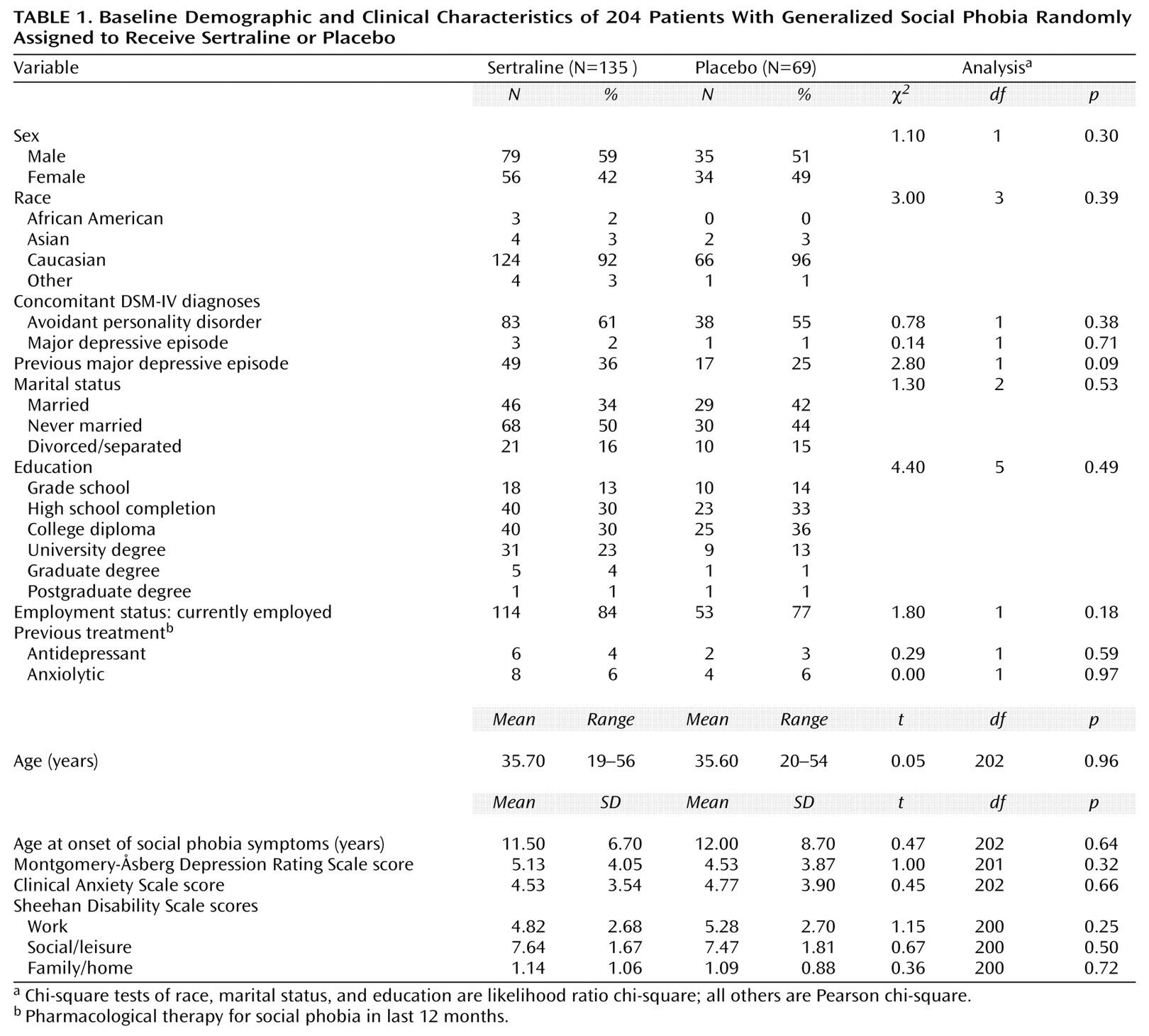
Two hundred four patients with generalized social phobia were enrolled and randomly assigned to receive either sertraline (N=135) or placebo (N=69). The patients’ baseline demographic and clinical characteristics are shown in
Table 1. Four patients (2%) had a comorbid episode of major depression at baseline. Mean Sheehan Disability Scale scores at baseline indicated moderately impaired work, markedly impaired social life/leisure activities, and mildly impaired family life/home responsibilities. There were no statistically significant differences between groups in demographic characteristics or mean baseline rating scale scores (
Table 1 and
Table 2).
Patient Disposition
Postbaseline safety assessments were obtained for all 204 patients in this study. One patient received sertraline but did not receive an efficacy evaluation after baseline. Therefore, the efficacy data for the remaining 203 patients (i.e., the intent-to-treat population) are reported. In the sertraline group, 104 (77%) of 135 patients completed the 20-week trial. In the placebo group, 54 (78%) of 69 patients completed the trial. The reasons for patient discontinuation in the sertraline and placebo groups, respectively, were adverse events (N=16 [12%] versus N=1 [1%]) (χ2=6.5, df=1, p<0.01), lack of efficacy (N=4 [3%] versus N=4 [6%]) (χ2=0.97, df=1, p=0.32), withdrew consent (N=4 [3%] versus N=7 [10%]) (χ2=4.6, df=1, p=0.03), lost to follow-up (N=3 [2%] versus N=1 [1%]) (χ2=0.14, df=1, p=0.71), protocol violation (N=1 [1%] versus N=0) (χ2=0.51, df=1, p=0.47), and administrative reasons (N=3 [2%] versus N=2 [3%]) (χ2=0.08, df=1, p=0.77).
Dose
At study endpoint, the mean dose of sertraline was 146.7 mg/day (SD=57.0, range=50–200); in the placebo group the endpoint dose was equivalent to 161.6 mg/day (SD=55.0, range=50–200). In sertraline patients who completed the study, the mean dose at endpoint for responders was 154.5 mg/day (SD=45.84, range=50–200 mg); for responders it was 179.7 mg/day (SD=39.89, range=50–200). The median duration of double-blind treatment was 140 days in both treatment groups.
Efficacy
Seventy-one (53%) of 134 patients receiving sertraline and 20 (29%) of 69 patients receiving placebo were much or very much improved at the end of treatment (χ
2=10.61, df=1, p<0.01) according to CGI improvement ratings. Forty (30%) of the patients receiving sertraline and nine (13%) of those receiving placebo were rated as very much improved at the end of treatment (χ
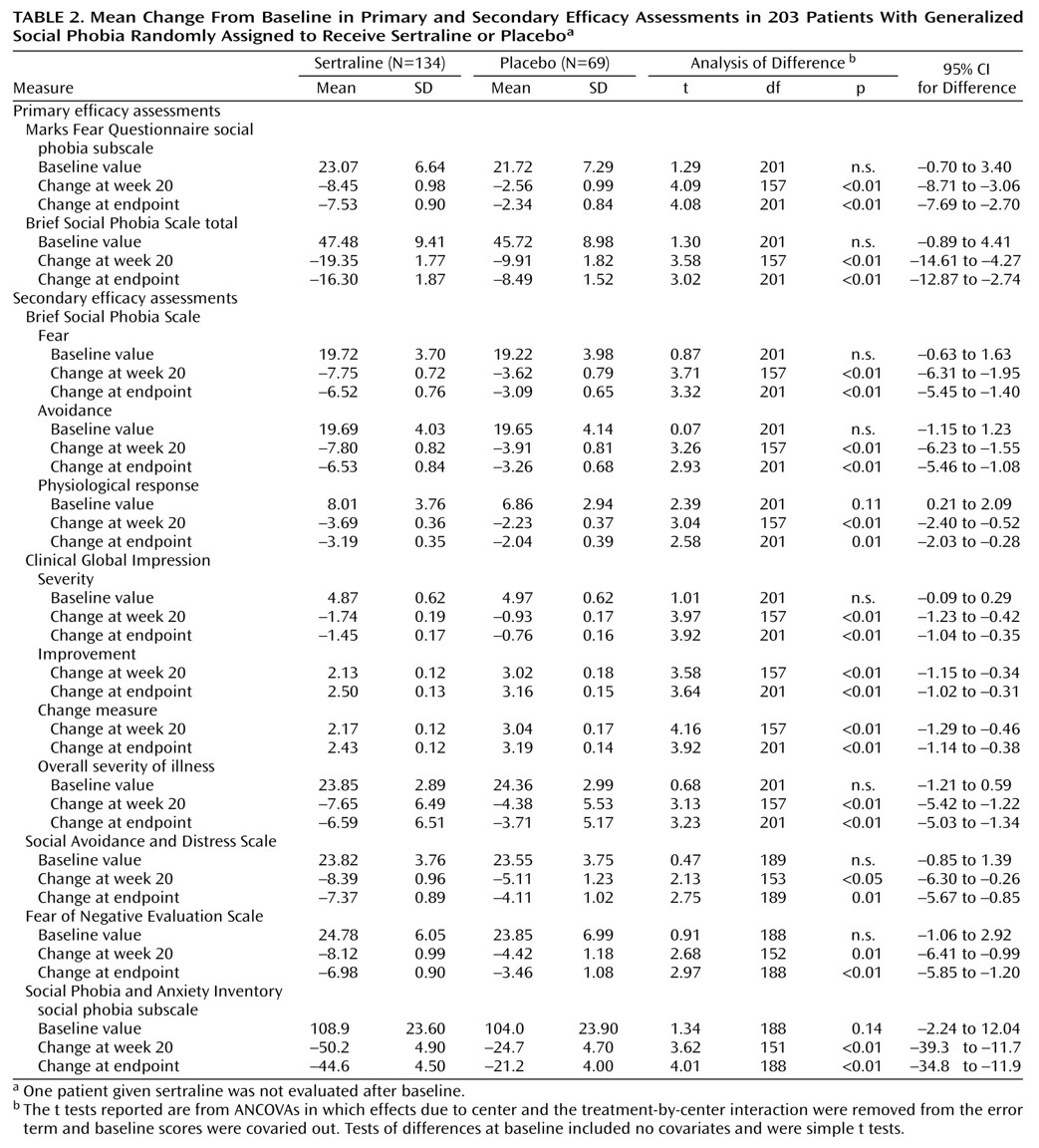
2=7.03, df=1, p<0.01) according to CGI improvement ratings. The mean total scores on the Marks Fear Questionnaire social phobia subscale and Brief Social Phobia Scale were reduced by 32.6% and 34.3% in the sertraline group and 18.8% and 18.6% in the placebo group, respectively (see corresponding mean change analyses in
Table2).
Sertraline-treated patients also demonstrated significantly greater improvements than patients receiving placebo on all secondary efficacy measures (
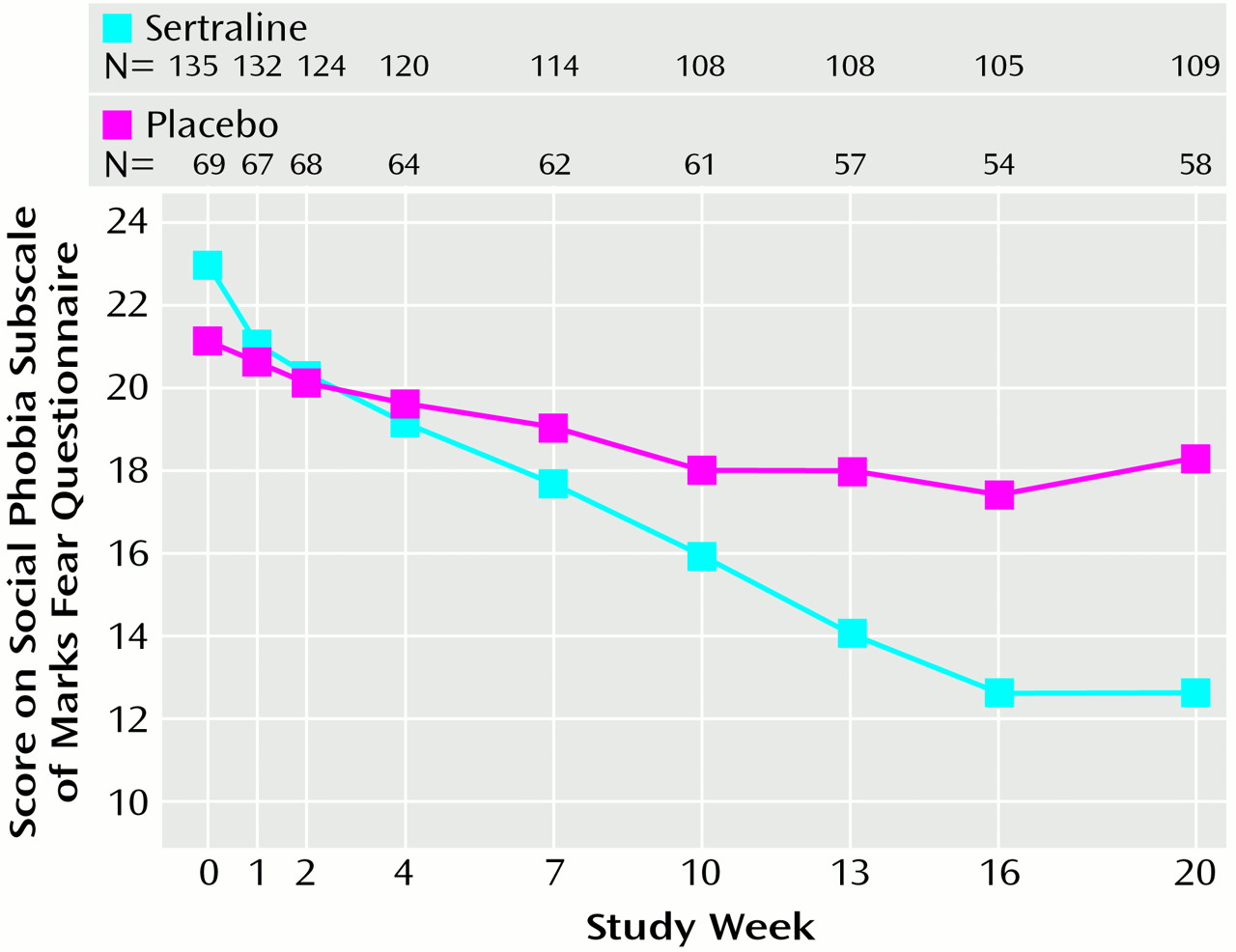
Table 2). No significant main effect of gender was found on treatment outcome, nor did gender interact with treatment group on any of the primary efficacy variables. The repeated measures analysis of variance (ANOVA) of the social phobia subscale of the Marks Fear Questionnaire across treatment, where baseline scores were entered as covariates, showed a significantly steeper improvement slope with sertraline than with placebo (F=8.1, df=7, 994, p<0.001).
Figure 1 presents the raw mean values for each time point associated with this analysis. Similar results were observed with repeated measures ANOVA of the Brief Social Phobia Scale data. Additional random regression analyses found significantly steeper improvement slopes in favor of sertraline for secondary efficacy measures.
Sertraline-treated patients who completed the 20-week study showed significantly greater improvements than placebo patients on all three subscales of the Sheehan Disability Inventory. At study endpoint, sertraline-treated patients improved significantly on the social life/leisure activities subscale of the Sheehan Disability Scale and nonsignificantly on the work and the family life/home responsibilities subscales. Random regression analysis of the changes from baseline showed significantly greater improvement on all three subscales of the Sheehan Disability Scale: work (t=–2.02, df=336, p<0.05, 95% CI=1.05–0.01), social life/leisure activities (t=–2.43, df=336, p<0.02, 95% CI=1.34–0.13), and family life/home responsibilities (t=–2.63, df=336, p<0.009, 95% CI=0.37–0.05).
Minimal levels of depressive symptoms on the Montgomery-Åsberg Depression Rating Scale and general anxiety symptoms on the Clinical Anxiety Scale were seen at baseline (
Table 1). Mean baseline Montgomery-Åsberg Depression Rating Scale total scores of 5.13 decreased to 4.42 at study endpoint in the sertraline group but increased in placebo-treated patients from 4.28 at baseline to 5.41 at study endpoint (F=7.5, df=1, 201, p<0.01). There was significant (p<0.05) correlation between improvement in Montgomery-Åsberg Depression Rating Scale scores and improvement of the primary efficacy assessments, but the correlation was weak (tau-b=0.2–0.29).
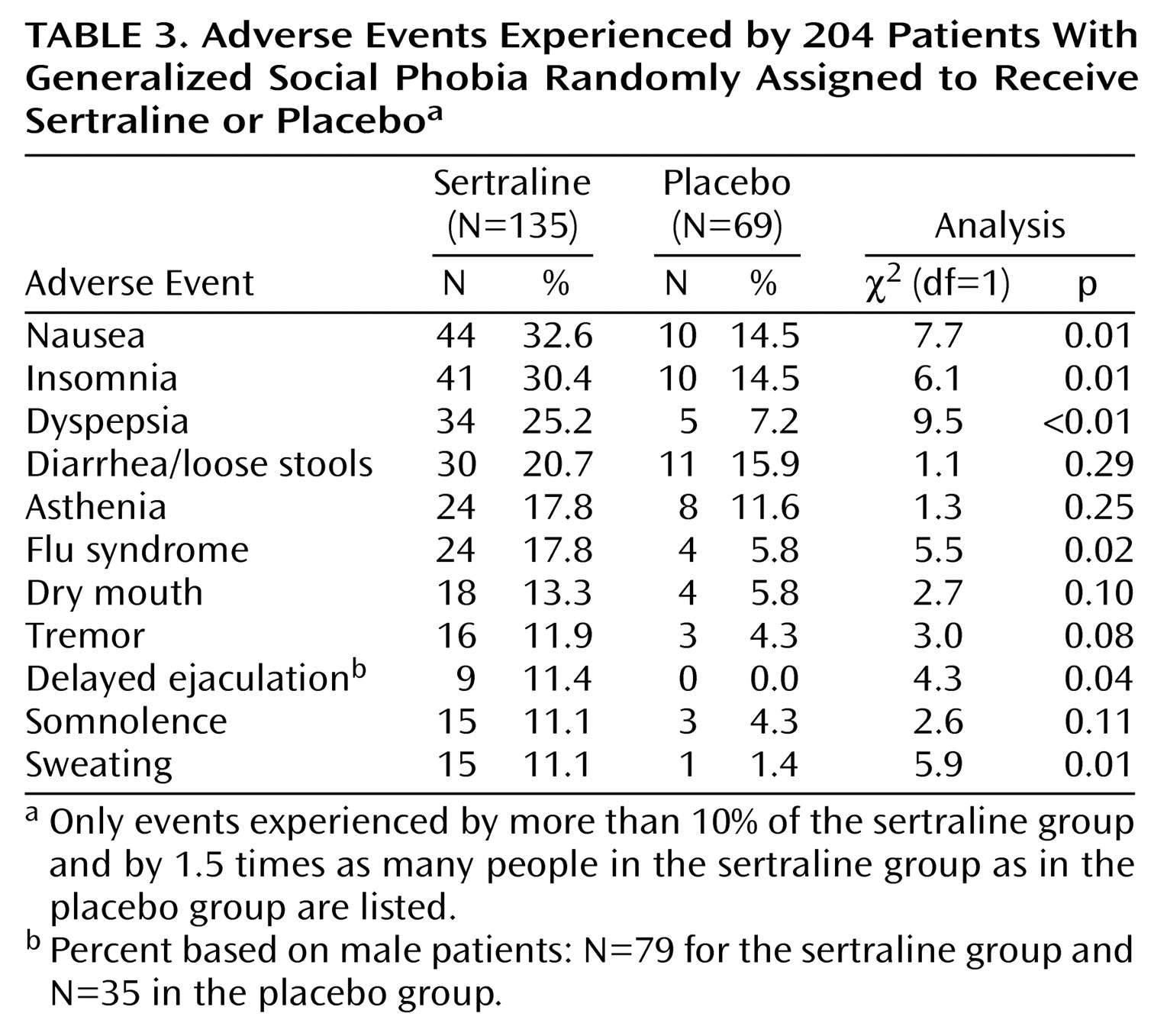
Safety
The reported incidence and severity of adverse experience seen in sertraline-treated patients were those expected with SSRI treatment (
Table 3). The incidence of nausea, insomnia, dyspepsia, flu syndrome, delayed ejaculation in males, and sweating were significantly more common in sertraline-treated patients than in placebo-treated patients. Only 7% of patients experienced severe adverse events in both the sertraline (N=10) and the placebo (N=5) groups. A higher percentage of sertraline-treated patients (12% [N=16]) than placebo-treated patients (1% [N=1]) discontinued treatment because of adverse experiences (χ
2=8.77, df=1, p<0.01). Nine patients (7%) in the sertraline group and four (6%) in the placebo group had their dose reduced or temporarily discontinued because of adverse experiences. There were no statistically significant differences between the sertraline and placebo groups in terms of changes in vital signs, including weight (all t<0.80, n.s.). One serious adverse event (e.g., life-threatening or resulting in hospitalization) was reported in a patient receiving sertraline, a 21-year-old male patient who exhibited paranoid preoccupations and flat affect after receiving the study medication for 47 days. This patient was hospitalized, withdrawn from the study, and responded to treatment with risperidone.
Discussion
This study demonstrates that sertraline is an effective and well-tolerated treatment in patients with generalized social phobia who, on average, had had symptoms of the illness for 24 years. The lack of direct comparative studies precludes between-treatment efficacy comparisons. Highly expert investigators at single-center sites, the use of differing eligibility criteria, and the use of differing definitions of study population for efficacy analysis mean that comparisons with data from other studies in the field are difficult. However, the rate of response to sertraline in this generalized social phobia study is comparable to that of other SSRIs in double-blind, multicenter, placebo-controlled studies
(16,
17).
Improvement in the placebo group appeared to plateau from weeks 7–10 onward (
Figure 1). In contrast, the improvements in the sertraline group gradually increased throughout the study. Statistically significant differences (p<0.05) between sertraline and placebo were present on several measures at week 7 and present on most study assessments for social phobia and on the subscales of the Sheehan Disability Scale at week 10. The gradual and progressive onset of therapeutic effect of sertraline found in this study is reminiscent of the response in obsessive-compulsive disorder. However, a contributing factor may be the very gradual dose titration in the study. The initial dose could not be increased for at least 4 weeks, and increases thereafter were at a minimum of 3-week intervals.
In addition to significant improvements in the symptoms of generalized social phobia seen consistently across a range of physician-rated and patient-rated scales, an improvement was also seen in patients’ work function. At baseline, patients rated themselves on average as having moderately impaired work functioning. In patients completing the study, those receiving sertraline reported mild impairment, in contrast to placebo-treated patients, who remained moderately impaired.
In conclusion, this controlled clinical study confirms the findings of earlier open and single-center, placebo-controlled studies that sertraline is an effective and well-tolerated treatment for patients with generalized social phobia. On the basis of previously published data and the efficacy, tolerability, and safety findings of the present 20-week study, sertraline can be considered among the first-line treatments for social phobia. Further research should address questions of optimal dose, the duration of treatment, and the potential usefulness of the addition or augmentation of cognitive behavior treatment to improve response and/or reduce relapse.