There is an increasing awareness of the presence of attention deficit hyperactivity disorder (ADHD) in adults. Despite controversy
(1), studies of clinical correlates, neuropsychology, familial aggregation, and neuroimaging have supported the validity of this disorder in adults
(2). Adults with ADHD have high rates of psychopathology, substance abuse, social dysfunction, and academic and occupational underachievement
(3–
5). Conversely, adults with ADHD are overrepresented among those seeking treatment for substance abuse
(6,
7) and depression
(8). Although ADHD was initially conceptualized as a childhood disorder, follow-up studies have documented that approximately one-half of affected youth continue to have ADHD into adulthood
(3,
9–
11). Although epidemiological data are limited, a relatively recent study suggests that up to 4.7% of adults may meet criteria for ADHD
(12).
Despite the emerging recognition of adult ADHD, there is a paucity of data on the treatment of this disorder
(13). For instance, in contrast to the more than 200 controlled studies of stimulant use in children with ADHD
(14–
16), we are aware of only nine controlled studies of the use of stimulants in adults with ADHD
(14). Although this work has demonstrated the efficacy of stimulants for the treatment of adult ADHD, the multiple daily doses, scheduled prescribing restrictions, anxiogenic properties, and liability for abuse limit their usefulness in treating subgroups of adults with ADHD
(14,
17). Moreover, the co-occurrence of mood and substance use disorders in patients with ADHD supports the development of safe and effective nonstimulant alternatives.
Tricyclic antidepressants and bupropion have emerged as second-line agents for treating pediatric ADHD
(16). Bupropion is a novel aminoketone antidepressant related to the phenylisopropylamines and pharmacologically distinct from available antidepressants
(18,
19). Bupropion has been shown in preclinical studies to manifest antidepressant properties with indirect dopaminergic and noradrenergic agonist effects, although the clinical relevance of these findings remains unclear
(19). Bupropion at doses of up to 6 mg/kg per day has been shown in controlled clinical trials in youth to be effective in reducing ADHD symptoms, albeit less robustly than stimulants
(16,
20–
22). Data on ADHD in adults, however, are restricted to one open trial of 19 adults treated with an average of 360 mg/day of bupropion for 6–8 weeks
(23). In this 1990 study, Wender and Reimherr
(23) observed that 74% of patients completing the trial manifested a positive and sustained response. Although this information was helpful, the high dropout rate (27%), the open nature of the study, and the recent availability of a sustained-release preparation of bupropion necessitate a reexamination of the role of this compound in the treatment of adults with ADHD. To this end, we conducted a placebo-controlled trial of the sustained-release preparation of bupropion in a well-characterized group of adults with ADHD. On the basis of the available pediatric and adult literature, we hypothesized that bupropion would be superior to placebo in the treatment of adults with ADHD.
Results
Of the 154 subjects screened, 40 (26%) subjects were enrolled in the study (30 were not interested, 27 did not return for follow-up, 17 had current substance abuse, 11 were receiving exclusionary psychotropics, 10 had no ADHD, nine had bipolar or psychotic disorder, six had medical contraindications, and four had previous exposure to bupropion). The final study group consisted of 18 women and 22 men who ranged in age from 20 to 59 years (mean=38, SD=11). Thirty-eight subjects completed the protocol; two subjects dropped out because of noncompliance (both receiving bupropion).
Demographics and Comorbidity
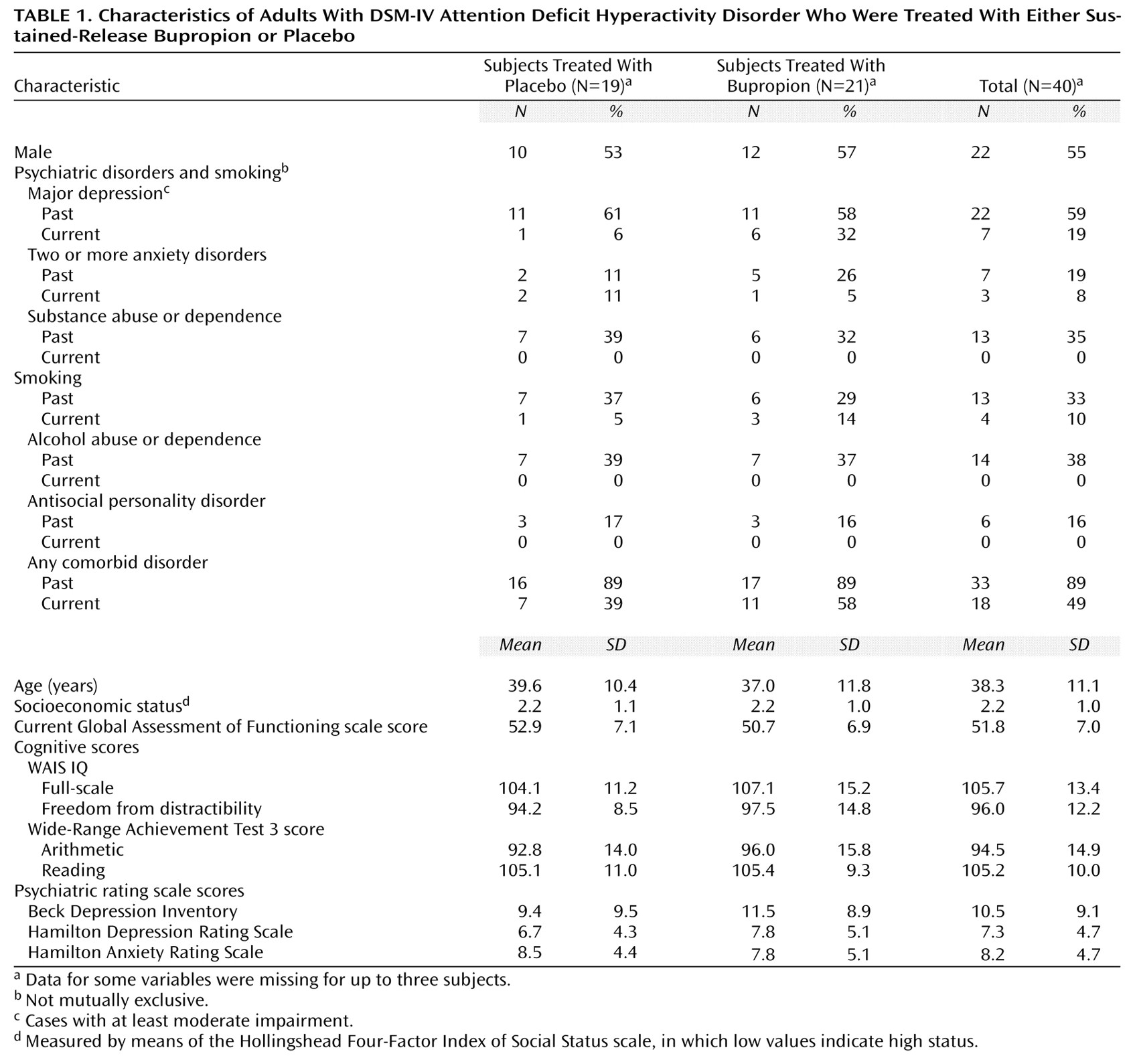
Subjects were most frequently diagnosed with the inattentive subtype of ADHD (N=23, 58%), followed by the combined (N=14, 35%) and hyperactive or impulsive subtypes (N=3, 8%). As depicted in
Table 1, 89% of the subjects with ADHD had at least one past comorbid psychiatric disorder; for 49% (data were missing for three subjects), the comorbid disorder was also present within the past month. The results did not differ significantly between the placebo and bupropion groups (past ADHD: p=1.00, Fisher’s exact test; current ADHD: p=0.33, Fisher’s exact test). Before entering this study, 11 subjects had been taking medications for ADHD, seven had received counseling, and seven had received both medication and counseling. Despite this group of adults with ADHD possessing average to above-average intelligence, 17 (46%) had required tutoring in school, and 11 (30%) had repeated at least one grade (some data were missing). The rate of past smoking status did not differ between the patients in the bupropion and placebo arms (p=0.74, Fisher’s exact test). Likewise, there were also no significant differences in terms of current smoking status (p=0.61, Fisher’s exact test).
Outcome Assessment
By using categorical definitions of ADHD improvement (with last observation carried forward), bupropion was found to be clinically and statistically superior to placebo in adult patients. By using a predefined criteria of a CGI improvement rating of 1 or 2 (“much improved” to “very much improved”), a significantly higher proportion of subjects were considered improved while receiving bupropion than while receiving placebo (N=11, 52%; N=2, 11%) (p=0.007, Fisher’s exact test). A similar result was obtained by using a preestablished definition of improvement of 30% or more reduction in scores on the DSM-IV ADHD symptom checklist (N=16, 76%; N=7, 37%) (p=0.02, Fisher’s exact test). The same pattern of results was observed when the group was stratified by past and current smoking status, although statistical significance was not reached because of reduced group size.
Although the subjects with ADHD who were randomly selected for the active treatment arm had a baseline mean score of 32.9 (SD=7.8, range=21–47) on the ADHD symptom checklist, week-6 endpoint analysis (with last observation carried forward) revealed a 42% reduction in scores (week 6: mean=19.2, SD=11.0, range=0–41). Comparatively, placebo group baseline scores (mean=31.3, SD=8.5, range=19–47) decreased by only 24% by week 6 (mean=23.8, SD=11.8, range=7–46), resulting in a significant difference between groups (t=–2.02, df=39, p=0.05, linear regression). Results from the generalized estimation equations model, using ADHD symptom checklist scores from all time points, indicated a significant effect of time (z=–4.66, p<0.001), no significant main effect of drug (bupropion or placebo) (z=0.69, p=0.49), and no drug-by-time interaction for ADHD symptoms (z=–1.29, p=0.20). The bulk of improvement in ADHD symptom profiles occurred in weeks 5 and 6.
We also evaluated the impact of treatment on the 18 DSM-IV specific symptoms of ADHD (with last observation carried forward). These analyses showed that compared to baseline, a significantly greater number of ADHD symptoms improved in subjects receiving bupropion compared to those receiving placebo: all 18 symptoms improved significantly in the bupropion-treated group, whereas only eight (44%) of 18 of the symptoms improved in the placebo group (p<0.001, Fisher’s exact test). Response to treatment was not significantly related to DSM-IV ADHD subtype (inattentive versus combined).
Baseline ratings of depression (mean Hamilton depression scale score and mean Beck Depression Inventory score) and anxiety (mean Hamilton anxiety scale score) were relatively low and did not differ between groups (all p>0.05, Wilcoxon rank-sum test). When using standard cutoff points for depression (Hamilton depression scale score: >16, Beck Depression Inventory score: >19, and CGI severity scale score: 4) and anxiety (Hamilton anxiety scale score: >21 and CGI severity scale score: 4), eight subjects had scores indicative of depression at baseline per the CGI severity scale (three taking placebo and five taking bupropion), five had scores above the Beck Depression Inventory cutoff (two taking placebo and three taking bupropion), and eight had scores indicative of anxiety per the CGI severity scale (three taking placebo and five taking bupropion). There was no significant medication effect (medication versus placebo at endpoint) on the Hamilton depression scale, Beck Depression Inventory, or Hamilton anxiety scale, including analyses of all subjects stratified by the presence of abnormal baseline scores (all p>0.05, Wilcoxon rank-sum test). There was no difference in ADHD symptom checklist scores or CGI ADHD scores (improvement or severity) in adults with past or current anxiety or major depression (placebo or bupropion group, p>0.05, Wilcoxon rank-sum test). Similarly, there was no effect of gender or socioeconomic status on response to bupropion, although we lacked adequate statistical power to fully evaluate the impact of treatment on comorbidity, socioeconomic status, or gender.
There was no relationship between response and bupropion daily dose (t=–0.11, df=19, p=0.91). Average daily doses of placebo and bupropion at the end of the trial (week 6) were 379 mg/day and 362 mg/day, respectively. At the conclusion of the study, 16 bupropion subjects (76%) were receiving the full dose of 400 mg/day, two (10%) were receiving 300 mg/day, and three (14%) were receiving 200 mg/day. A total of 57% (12 out of 21) of the bupropion responders opted to continue with bupropion treatment at the conclusion of the study.
Adverse Effects
No serious adverse drug effects were observed during the trial. Adverse effects reported in at least two (5%) of the subjects included headache (bupropion: 19%; placebo: 16%), gastrointestinal problems (19% versus 16%), insomnia (38% versus 16%), aches or pains (10% versus 5%), dry mouth (10% versus 0%), and chest pain (10% versus 0%). There were no statistically significant differences between the study groups in the rates of any single adverse event or in the rate of at least one adverse event (bupropion: N=14, 67%; placebo: N=11, 58%) (all p>0.05, Fisher’s exact test). Not including the two bupropion dropouts, five subjects taking bupropion and three subjects taking placebo lowered their dose because of adverse effects.
Evaluation of vital signs failed to reveal any differences between the subjects in the bupropion and placebo arms. Specifically, there were no statistically significant effects of bupropion compared to placebo on heart rate at week 6 (mean=78.4, SD=14.4; mean=72.7, SD=12.0, respectively) (z=–1.35, p=0.18, Wilcoxon rank-sum test). Likewise, there were no significant differences between the treatment groups at endpoint on systolic (mean=127.9, SD=13.2; mean=124.6, SD=18.2) (z=–0.83, p=0.41, Wilcoxon rank-sum test) or diastolic (mean=73.5, SD=10.2; mean=73.1, SD=9.0 (z=–0.15, p=0.88, Wilcoxon rank-sum test) blood pressure.
Discussion
In this randomized, double-blind, placebo-controlled trial, our results demonstrated the clinical efficacy and tolerability of sustained-release bupropion for the treatment of ADHD in adults. By clinical impression, 52% of adults with ADHD who received bupropion were considered “much improved” to “very much improved,” whereas only 11% of those receiving placebo were so classified (p=0.007, Fisher’s exact test). This modest therapeutic effect was seen after several weeks, which suggests an apparent delayed onset of action in these adults with ADHD.
The current results confirm and extend previous open findings in adults
(23) and adolescents
(41), as well as controlled studies in juveniles
(20–
22), that found bupropion to be effective in reducing ADHD symptom profiles. Our response rate (30% or more reduction in ADHD symptom checklist score) is identical to that reported in an open study by Wender and Reimherr
(23) using the immediate-release preparation of bupropion. Moreover, the magnitude of response observed in the current study is similar to that found in previous controlled investigations in children and adolescents with ADHD employing similar weight-corrected doses of bupropion
(20–
22). Hence, as in results found in children and adolescents, our data indicate that adults with ADHD respond favorably to bupropion treatment.
The relatively low placebo response noted in the current study is consistent with data from our previous studies documenting the low placebo response in adult ADHD
(26,
27,
32,
42). The 52% response rate (per the CGI scale) observed with bupropion in this study was somewhat lower than the response rate observed in our prior, methodologically similar trials of methylphenidate (87%)
(26), desipramine (89%)
(27), and amphetamine compounds (75%)
(43). However, the response rate to bupropion was similar to that achieved with pemoline (50%)
(42), an experimental cognition-enhancing agent (40%)
(33), and the nonstimulant investigational agent tomoxetine (52%)
(32). Hence, given the current results, bupropion appears to follow stimulants and desipramine in terms of efficacy for treating ADHD in adults. It remains unknown whether a longer study at higher doses could lead to better results. The study was only 6 weeks long; that may have been insufficient time for the full clinical benefit of bupropion to unfold. In support of this notion, our data suggest that the therapeutic value of bupropion was most striking in the final 2 weeks of the study, after the subjects had achieved their highest dose of bupropion. Our current data mirror previous data with desipramine in adults with ADHD, which indicated a delayed onset of maximal efficacy, with the largest improvement occurring after the dose was maximally titrated (i.e., between the 2-week end of titration and 6-week endpoint)
(27).
This study was not a dose-response evaluation; our results did not identify statistically significant associations between clinical effect and bupropion dose, which is consistent with findings in pediatric studies of bupropion and other antidepressants
(16). Consistent with our previous controlled studies in adults with ADHD, response to bupropion was not affected by gender or social class. Moreover, our lack of a significant association of past or current depression or anxiety influencing ADHD symptoms suggests that bupropion is effective in the presence of anxiety or depression in reducing the symptoms of ADHD.
As part of its mechanism of action, bupropion has been shown to potentiate dopaminergic neurotransmission
(19). The current findings support the notion that pharmacological agents that are effective in reducing ADHD symptoms have similar catecholaminergic properties
(16,
44). Agents such as stimulants and antidepressants appear to facilitate directly norepinephrine and dopamine neurotransmission, whereas nicotinic cognitive enhancers may indirectly affect such systems
(33,
45,
46). For example, research suggests that recently described polymorphisms in the postsynaptic D
4 receptor in youth
(47) and adults
(48) with ADHD may result in a blunted response to dopamine
(49). If substantiated, these findings would further the hypotheses linking ADHD with catecholaminergic dysregulation in general and dopaminergic systems in particular
(44).
The results of this study should be viewed in light of its methodological limitations. Only 26% of the subjects screened were enrolled in the study. The majority of subjects were from relatively high socioeconomic strata; hence, the results of the current study may not generalize to lower socioeconomic strata. Despite subjects meeting criteria for a lifetime diagnosis of depression or anxiety per structured psychiatric interview, the majority had low current scores on depression and anxiety rating scales, which limited our ability to evaluate the efficacy of bupropion in these comorbid conditions. Other limitations included the use of a relatively short exposure to a full dose of medication, which may not have allowed adequate time for the full therapeutic benefit of bupropion treatment to emerge.
Although our results are based on self-reports from affected individuals, it has been suggested that subjects with ADHD may not be ideal reporters of their disorder
(29). Although this places some limits on the interpretation of our results, the significant effects on ADHD symptoms observed in this and previous studies
(26,
27,
32,
33,
42,
50) suggest that adults with ADHD are acceptable reporters of their own condition. In addition, self-reports of ADHD symptoms have been shown to be a reliable and valid method of assessing ADHD in adults
(51,
52).
Despite these limitations, the results of this study show that bupropion significantly improved ADHD symptoms in adults. Bupropion may have a delayed onset of action of from 4 to 6 weeks in treating ADHD. Given that it has less efficacy for ADHD compared to stimulants
(26,
43), bupropion appears to be useful as a second-line agent for the treatment of uncomplicated ADHD in adults. However, because of its freedom from the liability of abuse, bupropion may be considered a first-line therapy in special groups of individuals with ADHD, such as those with substance abuse
(53) or co-occurring prominent mood lability
(54). Since some stimulants (methylphenidate, pemoline, and amphetamine compounds) and some antidepressants (desipramine and bupropion) have now been shown in controlled trials to be effective in treating both pediatric and adult forms of ADHD, the present results further support the validity of ADHD in adults and the continuity of treatment responsivity across the lifespan.