Cerebellar abnormalities, including irregular Purkinje cell arrangement and loss, were described in patients with schizophrenia as early as 1959
(1). More recent histological evidence has been inconclusive
(2,
3), suggesting that marked Purkinje cell abnormalities are not the hallmark of cerebellar pathology in schizophrenia. Computerized tomography studies have provided data showing structural abnormalities, particularly vermal atrophy, in the cerebella of patients with schizophrenia
(4,
5). Experiments applying magnetic resonance imaging (MRI) techniques have been inconclusive, some finding a greater vermal volume in patients with schizophrenia
(6), others reporting no differences
(7,
8), and still others finding smaller cerebellar volume in patients
(9).
The current investigation examined volume measurements of individual cerebellar regions in patients with schizophrenia. We hypothesized that cerebellar regions connected with cortical regions associated with the pathophysiology of schizophrenia would have abnormal volumes. Furthermore, on the basis of the prevalence of cerebral asymmetry in schizophrenia
(10), we hypothesized that the cerebellar hemispheres would exhibit a reversed pattern of asymmetry.
Method
Nineteen subjects with schizophrenia blindly diagnosed according to DSM-III-R (15 men and four women; mean age=34 years, SD=6), and 19 psychiatrically well comparison subjects (15 men and four women; mean age=27, SD=6) participated in the study. Comparison subjects had no personal or family history of neurological illness and no axis I disorder. Other exclusion criteria for both patients and comparison subjects included any history of organic brain syndrome, head injury, or motor disturbance. The study was approved by the McLean Hospital Institutional Review Board. Written informed consent was obtained from all participating subjects.
MRI data were acquired with a 1.5-T GE Signa scanner (GE Medical Systems, Milwaukee) retrofit with an Advanced NMR Systems (Woburn, Mass.) whole-body echo-planar imaging coil. A spoiled gradient-recalled acquisition of T1-weighted images was used (TR=35 msec, TE=5 msec) with 1.5-mm thick contiguous coronal slices.
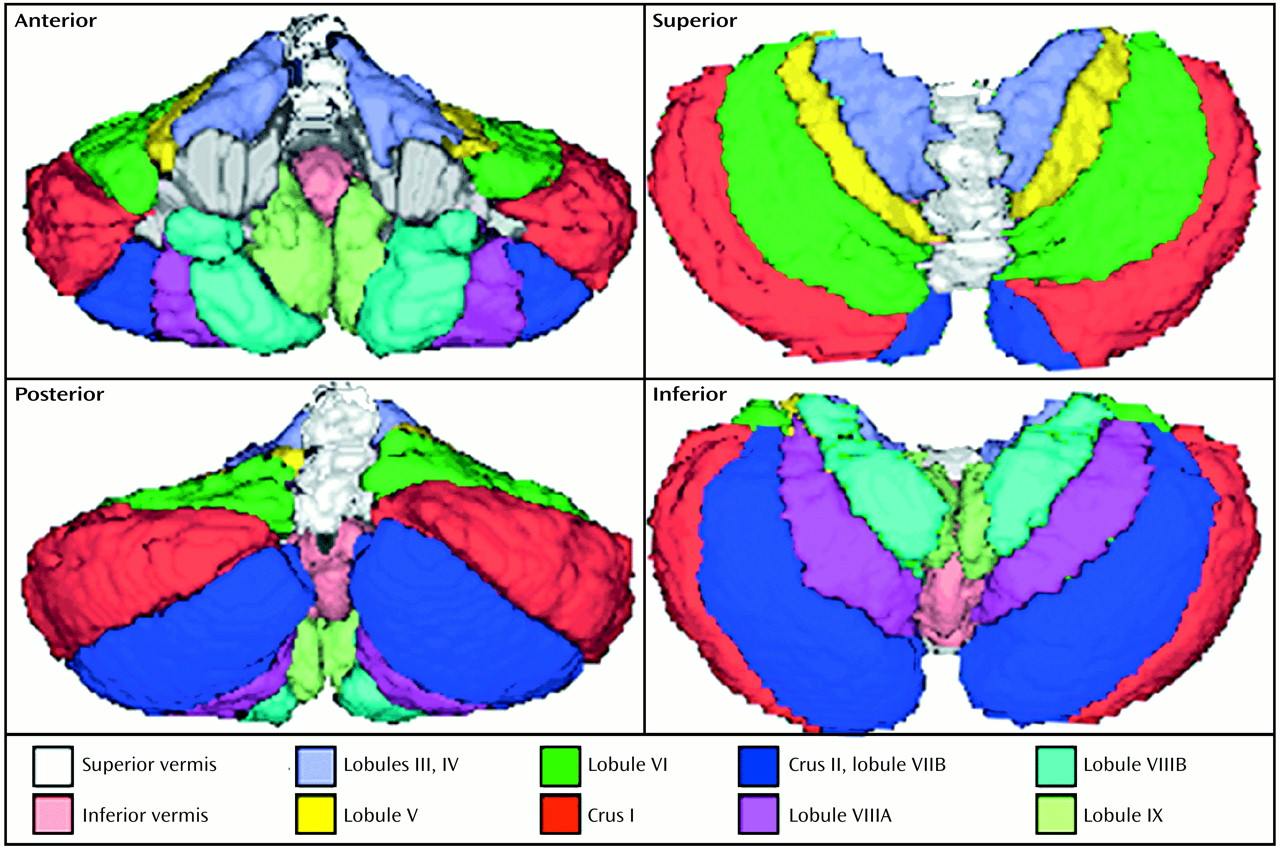
Images were filtered and segmentation was completed by applying a method based on visual inspection. Parcellation of the cerebellum was carried out with the aid of the high-resolution MRI cerebellar atlas of Schmahmann et al.
(11). The cerebellum was divided into 18 individual regions. Three-dimensional reconstructions are shown in four orthogonal views in
Figure 1 to demonstrate the divisions made by tracing the fissures. The volume of each region was calculated as the sum of all pixels within the region across slices multiplied by the slice thickness and voxel aspect ratio. Reliability of these measures was confirmed by calculating the intraclass correlation coefficient for all regions of 10 subjects (r=0.95 for all regions; range=0.92–0.99). Lateralization of cerebellar hemisphere volume was examined as an index of asymmetry: (right–left)/(right+left). Following the analysis of this index, one patient with schizophrenia was dropped from all tests because of a value outside of two standard deviations from the mean.
Statistical analyses were performed with StatView (Abacus Concepts, Inc., Berkeley, Calif.). Differences between diagnostic groups in morphometric measures were analyzed by using analysis of covariance with total cerebral volume as the covariate. Indexes of asymmetry were compared by using a one-tailed Student’s t test because the direction of the change was hypothesized a priori. We hypothesized that differences in asymmetry would follow the pattern frequently observed for the cerebral cortex. Bonferroni correction was not performed.
Results
The mean inferior vermal volume was smaller in the patients with schizophrenia than in the comparison subjects (F=5.32, df=1, 33, p<0.03). Although the differences between groups in mean superior vermal volume were not significant, total vermal volume was significantly smaller in the patients with schizophrenia (F=4.56, df=1, 33, p<0.05). The groups did not differ significantly in other regional mean volume measures or in total cerebellar volume.
Measures of laterality revealed a right-greater-than-left hemispheric volume asymmetry for both groups. However, this asymmetry measure was significantly smaller in the patients with schizophrenia (for comparison subjects, mean=1.73, SD=0.99; for patients with schizophrenia, mean=0.82, SD=1.23) (t=2.47, df=35, p<0.01).
Discussion
This study found a smaller inferior vermal volume and total vermal volume in patients with schizophrenia than in comparison subjects without schizophrenia. Developmental studies have found that granule cell production in vermal lobules VI–VIII follows that of the rest of the vermis and that lobule VIII is the only vermal region to develop third-order sublobules, suggesting that cell production and volume expansion occur later and to a greater extent in the inferior vermis
(12). Studies documenting smaller vermal volumes in childhood-onset schizophrenia
(13), fragile X syndrome
(14), and autism
(15) have also implicated cerebellar changes as part of an anomalous developmental process. The current study findings are consistent with the perspective that the inferior vermis, a large part of which is composed of lobule VIII, may be associated with an anomalous developmental process in schizophrenia.
This study also found less laterality in the cerebellum of patients with schizophrenia than in normal subjects. The abnormal pattern of cerebellar asymmetry may be related to changes in the cerebral cortex. Apart from the change in inferior vermal volume, we did not observe focal morphologic changes, suggesting that the difference in overall cerebellar asymmetry in schizophrenia may be due to a subtle and diffuse loss of inputs from the left cerebral cortex. Altered cerebral asymmetry in schizophrenia is postulated to result from a primary genetic defect
(16). The present study provides evidence that anomalous cerebellar asymmetry is also related to schizophrenia.
In conclusion, cerebellar changes that accompany schizophrenia appear localized to the vermis but are not regionally specific within the hemispheres. Vermal changes are consistent with earlier findings regarding the importance of this structure for emotion modulation and integration of information from both cerebral hemispheres. A relative loss of normal cerebellar asymmetry in the schizophrenic group may be related to differences in cortical asymmetries in these patients. The observed morphologic changes may result from the combined or individual influence of genetic predispositions or nonfamilial factors, such as early brain insults.