Our review of the literature on the pathophysiologic basis of comorbid PTSD and addiction selectively focuses on studies of the hypothalamic-pituitary-adrenal (HPA) axis and the noradrenergic system, as these have been most extensively studied in PTSD. It must be emphasized that many other neurobiological systems are involved in both the acute and chronic adaptation to stress and to substance use. These systems include the dopaminergic, γ-aminobutyric acid, benzodiazepine, and serotonergic systems, as well as the thyroid axis. Interactions among these systems in patients with comorbid PTSD and substance dependence are enormously complex. Thus, the potential relationships we discuss between the HPA axis, the noradrenergic system, and symptoms in patients with comorbid PTSD and substance use disorders should be viewed as one part of a far more complex whole.
HPA Axis in PTSD and Addiction
In humans and animals, acute stress elicits a cascade of neurohormonal events, including increased turnover of norepinephrine in terminal projection regions of the locus ceruleus and liberation of hypothalamic corticotropin-releasing hormone (CRH) into the pituitary portal system, which stimulates release of ACTH from the pituitary, which in turn triggers release of cortisol (human) or corticosterone (rat) from the adrenals
(20). Animal and human research has implicated this cascade in the pathophysiology of both substance use disorders and PTSD.
Humans with substance dependence most frequently identify stress and negative mood states as reasons for relapse and ongoing substance abuse
(21). Recently, a personalized stress imagery task was shown to reliably increase cocaine craving and salivary cortisol in cocaine-dependent patients
(22). Animal studies have shown that stress induces relapse to heroin and to cocaine self-administration in rats trained to self-administer these substances and then subjected to a prolonged drug-free period
(23,
24). Similarly, in animals naive to illicit substances, a large range of stressors increases the proclivity toward drug self-administration
(25). Initial work on the pathophysiology of this phenomenon indicated that stress-induced or stress-enhanced drug self-administration is mediated by corticosterone
(26).
Evidence has accumulated to support a role for CRH in mediating the effects of stress on drug self-administration. Central, but not peripheral, administration of CRH has been shown to induce a long-lasting enhancement (sensitization) of the locomotor response to d-amphetamine
(27), and pretreatment with a CRH antagonist has been shown to block the development of stress-induced sensitization to d-amphetamine
(28). Indeed, central administration of anti-CRH antibody or the CRH receptor antagonist α-helical CRH has been found to block the locomotor hyperactivity induced by cocaine
(29).
Withdrawal from chronic cocaine or alcohol administration in rats produces anxiety-like behavior and decreased exploration that is associated with selective increases in CRH in the hypothalamus, amygdala, and basal forebrain
(30,
31). Pretreatment with anti-CRH immunoserum or α-helical CRH, blocking the effects of CRH, completely prevents the development of these withdrawal-associated behaviors
(30). Consistent with these observations, CSF CRH is elevated in humans in acute alcohol withdrawal and then normalizes or decreases below normal levels with extended abstinence and resolution of withdrawal symptoms
(32). Shaham and colleagues
(33) found that intracerebroventricular injection of CRH reinstated heroin seeking after extinction in rats trained to self-administer the drug. In addition, α-helical CRH attenuated the reinstatement effect of footshock stress
(33). Neither adrenalectomy nor chronic or acute exposure to the corticosterone synthesis inhibitor metyrapone interfered with the reinstatement effects of priming injections of heroin or of footshock stress. A potent, selective CRF1 receptor antagonist, CP-154,526, has been found to attenuate reinstatement of drug seeking induced by footshock stress after up to 14 days of extinction in rats trained to self-administer heroin or cocaine
(34).
Findings from both animal and human studies of the effects of chronic stress or of PTSD on HPA axis function vary depending on the experimental paradigm used or the population studied. In patients with PTSD, elevated
(35), reduced
(36), and normal
(37) levels of cortisol secretion have been reported. A series of studies performed by Yehuda and colleagues demonstrated that patients with PTSD have an elevated number of lymphocyte glucocorticoid receptors
(38), enhanced suppression of cortisol after administration of dexamethasone
(39), a greater than normal decrease in the number of lymphocyte glucocorticoid receptors after administration of dexamethasone
(39), and higher than normal increases in ACTH after metyrapone blockade of cortisol synthesis
(40). All of these findings suggest that glucocorticoid negative feedback is enhanced in PTSD.
Animal studies examining the effects of uncontrollable stress on HPA axis function have reported initial increases of corticosterone secretion, followed by normalization of corticosterone secretion with ongoing chronic stress
(41). However, some investigators have failed to demonstrate normalization of corticosterone secretion with chronic uncontrollable stress
(42), particularly in animals that have been reared under stressful conditions
(43) or when levels of chronic stress are high
(44). In a pattern similar to that found in humans with PTSD, animals subjected to a single episode of prolonged stress and then briefly restressed after a stress-free period showed enhancement of glucocorticoid negative feedback
(45).
Although both animal and human studies have suggested that glucocorticoid negative feedback may be enhanced in PTSD, the implications of these observations for CRH secretion in this disorder are unclear. As noted earlier, CRH-producing cells and CRH receptors exist both in the hypothalamus and in extrahypothalamic sites. Findings from some studies have suggested that hypothalamic and extrahypothalamic CRH-producing cells may respond differently to corticosterone. Specifically, corticosterone appears to restrain hypothalamic CRH-producing cells while stimulating extrahypothalamic CRH-producing cells, particularly those in the amygdala
(46). Replacement of corticosterone in adrenalectomized rats decreases CRH production in the parvocellular nucleus of the hypothalamus while increasing CRH production in the central nucleus of the amygdala
(47). This region-specific pattern of regulation is also seen in adrenally intact rats treated with high-stress levels of corticosterone for extended periods of time
(48). Thus, while glucocorticoid feedback may decrease CRH production and release in the hypothalamus, it may stimulate CRH production and release in other brain regions, including the amygdala. This possibility has been addressed in two studies of patients with PTSD, one that examined CSF concentrations of CRH at a single time point
(49) and one that examined CSF concentrations of CRH at serial time points over a 6-hour period
(37). Both found significantly higher levels of CSF CRH in patients with PTSD than in normal comparison subjects. However, although elevated CSF CRH suggests that brain CRH may be elevated, the specific brain tissues producing CRH elevations cannot be determined from CSF data alone.
The possibility that brain CRH levels are elevated in PTSD is of great interest because of a rich preclinical literature indicating that elevated levels of CRH in the brain, particularly in the amygdala, potentiate fear-related behavioral responses, including the startle response
(50). These anxiogenic effects of CRH are reversed by administration of CRH antagonists
(50). As noted earlier, findings from animal and human studies have supported a role for CRH in mediating some effects of drugs of abuse, including stress- or priming-induced relapse to drug self-administration and symptoms of withdrawal
(27,
28,
32–
34). Thus, elevated levels of CRH in the brain in PTSD may mediate both the symptoms of hyperarousal as well as the increased risk for substance abuse and dependence seen in this disorder. More specifically, elevated levels of CRH in the brain in PTSD may enhance the euphorigenic properties of certain drugs, such as stimulants, and may worsen the severity of withdrawal symptoms, thereby prompting patients to relapse to drug use. Conversely, brain CRH elevations induced by withdrawal from substance use may exacerbate symptoms of hyperarousal, which could trigger other symptoms of PTSD, prompting relapse to substance use.
Noradrenergic System in PTSD and Addiction
During chronic uncontrollable stress, norepinephrine turnover increases in specific brain regions, including the locus ceruleus, hypothalamus, hippocampus, amygdala, and cerebral cortex
(51). Evidence for noradrenergic dysregulation in patients with PTSD has included elevated 24-hour urinary epinephrine and norepinephrine excretion, a lower than normal number of platelet α
2-adrenergic receptors, elevated 24-hour plasma norepinephrine, and exaggerated cardiovascular and 3-methoxy-4-hydroxyphenylglycol (MHPG) (a norepinephrine metabolite) responses to intravenous yohimbine
(52). Noradrenergic dysregulation has also been reported during states of withdrawal from chronic self-administration of alcohol and other abused substances. The levels of noradrenaline, norepinephrine, and MHPG in both plasma and CSF have been found to be increased and the number of platelet α
2-adrenergic receptors decreased in alcoholics during acute withdrawal
(53,
54). The severity of alcoholic withdrawal symptoms has been positively correlated with the concentration of MHPG in CSF
(54). Evidence for noradrenergic dysregulation in opiate withdrawal has included findings of elevated plasma MHPG in humans and elevated plasma and brain MHPG in animals
(55,
56). In animals, the level of noradrenergic activity was significantly correlated with the severity of withdrawal symptoms
(56). These findings have prompted the use of the α
2-adrenergic receptor agonist clonidine in the treatment of both opiate withdrawal symptoms and PTSD
(57,
58).
Noradrenergic System/HPA Axis Interactions
Evidence that brain CRH and noradrenergic systems modulate each other has been reported. Stress has been shown to increase CRH levels in the locus ceruleus
(59), a primary source of noradrenergic projections to all cortices as well as to the thalamus and hypothalamus, while intraventricular administration of CRH has been found to increase the discharge rates of locus ceruleus neurons and to increase norepinephrine turnover in hippocampus, hypothalamus, and prefrontal cortex
(60–
62). Conversely, stress-induced activation of the locus ceruleus has been blocked by administration of CRH antagonists
(63). Similar evidence exists for the interaction of the CRH and noradrenergic systems in the hypothalamus
(64) and the amygdala, where stress induces increases in both CRH and norepinephrine
(65). Furthermore, norepinephrine in the amygdala appears to stimulate release of CRH
(66).
These observations have prompted the proposal by Koob
(20) that interactions of the CRH and noradrenergic systems in the brain may, under some conditions, function as a feed-forward system, leading to the progressive augmentation of the stress response with repeated stress exposure that is characteristic of PTSD. This progressive augmentation of response with repeated stress has previously been conceptualized as kindling
(67). A feed-forward interaction between the CRH and noradrenergic systems may represent one neurobiologic underpinning of both PTSD and substance use disorders. More specifically, stress, including stress related to self-administration of or withdrawal from substances, may stimulate CRH release in the locus ceruleus, leading to activation of the locus ceruleus and release of norepinephrine in the cortex, which in turn may stimulate the release of CRH in the hypothalamus and amygdala
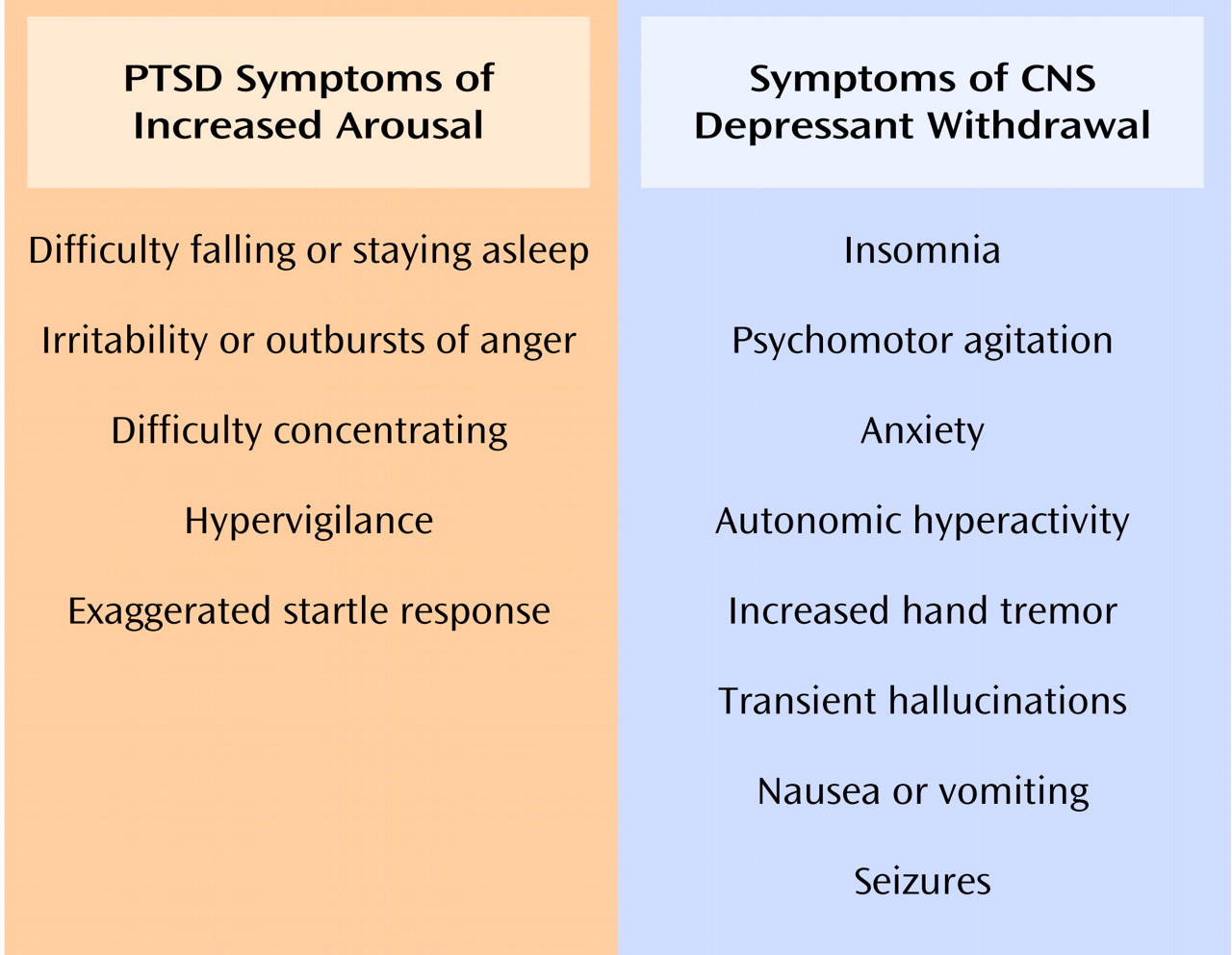
(20). Such an interaction between the brain noradrenergic and CRH systems may mediate the symptoms of hyperarousal seen in PTSD, including exaggerated startle response. The proclivity toward misuse of CNS depressants by patients with PTSD may reflect an attempt to interrupt this feed-forward interaction by suppressing activity of the locus ceruleus with these agents
(68).