Cancer patients, who experience marked physiologic, economic, and psychological stressors, have long been observed to have elevated prevalence rates of major depression, compared with the general population
(1). Among cancer patients, the highest prevalence rates are exhibited by patients with either oropharyngeal cancer
(2–
4) or pancreatic cancer
(5–
7). Prospective or cross-sectional studies of patients with pancreatic cancer have documented a remarkable point prevalence rate of depression of 50%
(5–
8). Although a number of neurochemical, neuroendocrine, neuroanatomical, and neuroimmune alterations have been associated with unipolar depression, few of these psychobiologic alterations have been systematically investigated in cancer patients with depression.
Increasing evidence has suggested that cytokines may play a role in the pathophysiology of mood disorders. Cytokines are intercellular signaling polypeptides produced by activated cells that regulate immune responses, the acute phase reaction, and hematopoiesis and play a central role in host defense
(9,
10). Cytokines also communicate information regarding immune activity to the brain and neuroendocrine system
(11). This cytokine-mediated “communication” between the immune and neuroendocrine systems
(12) is exemplified by the increased pituitary and adrenocortical secretion that occurs in response to infection or inflammation. Indeed, circulating cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor (TNF) have the capacity to directly stimulate the hypothalamic-pituitary-adrenal (HPA) axis and the release of corticotropin-releasing factor (CRF)
(11–
13). Of relevance to mood disorders, these cytokines can also induce “sickness behavior,” which includes symptoms of fatigue, anorexia, anhedonia (loss of interest in usual activities), decreased psychomotor activity, and disappearance of body care activities
(14,
15). These signs and symptoms accompany the immunologic response to infection and overlap with the symptoms of major depression. As a key regulator of the acute phase response
(16,
17), IL-6 has received special attention in a number of studies that have documented higher than normal acute phase reactants in depressed patients
(18,
19). Moreover, an increasing body of literature has documented the myriad neurophysiological effects of IL-6, IL-6 expression in human astrocytes and microglia
(20), and the region-specific distribution of IL-6 and IL-6 receptor mRNA in rat neurons within the medial preoptic nucleus, hippocampus, striatum, and medial hypothalamus
(21–
25).
To our knowledge, there have been no studies measuring IL-6 and its relation to major depression and HPA axis function in patients with cancer. In an effort to understand the pathophysiology of major depression in patients with cancer, we sought to determine whether cancer patients with comorbid major depression exhibited higher than normal plasma concentrations of the proinflammatory cytokine IL-6 and whether alterations in IL-6 were associated with perturbations of the HPA axis, i.e., a higher than normal level of cortisol in response to dexamethasone administration.
Method
Subjects
Study subjects were recruited among outpatients and inpatients at hospitals affiliated with Emory University and from the community by means of newspaper advertisements or word of mouth. The advertisements sought “cancer patients with and without depression” and “patients with depression.” After the study had been fully explained, 115 potential subjects gave written informed consent and were then screened by office interview for the presence of psychiatric disorders, substance abuse, medical illness, and medication intake. Subjects were excluded if they showed evidence of current alcohol or substance abuse, bipolar disorder (mania, hypomania, or cyclothymia), schizoaffective disorder, or schizophrenia. Subjects were between the ages of 18 and 80, had a Mini-Mental State Examination score of at least 24
(26), and had no untreated endocrine, cardiovascular, hematologic, hepatic, renal, or neurological diseases. Subjects were not receiving any psychotropic medications for treatment of depressive or anxiety symptoms. The normal comparison subjects were without any current or past personal history of psychiatric disorder. The depressed subjects without cancer and the normal comparison subjects were without significant medical illness, on the basis of findings of a physical examination, blood and urine analyses, ECG, and chest X-ray.
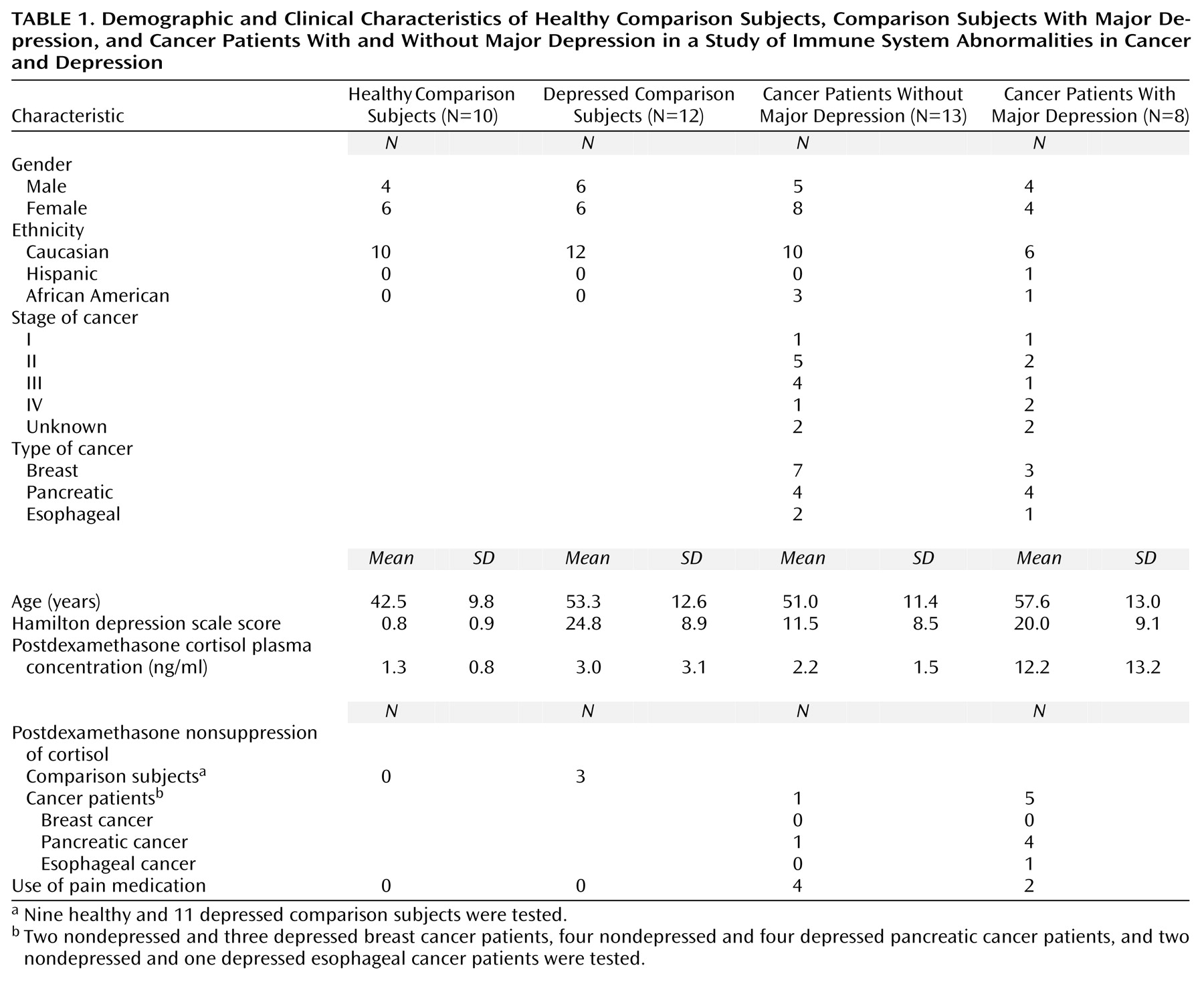
Of those screened, 10 healthy comparison subjects, 12 depressed subjects without cancer, and 21 cancer patients completed the study (
Table 1). This study was approved by the Emory University Human Investigational Committee, and informed consent was obtained from all subjects.
Procedure
All participants were interviewed with the Structured Clinical Interview for DSM-III-R (SCID)
(27) and completed a series of observer-rated and self-rated scales, including dimensional measures of depressive symptoms, the 21-item Hamilton Depression Rating Scale
(28), and the Carroll Rating Scale for Depression
(29). Further information regarding the mood and behavior of the cancer patients was obtained from family members. Final psychiatric diagnoses were provided by consensus of the research team (a master’s-level nurse, a research coordinator, a fourth-year psychiatry resident, and two board-certified psychiatrists) according to Spitzer’s procedure for longitudinal evaluation of all available data
(30).
Plasma IL-6 concentrations were obtained at 4:00 p.m. in all subjects and were measured with enzyme-linked immunosorbent assay by using sets of paired monoclonal antibodies for capture and detection, as suggested by the manufacturer’s protocol (Endogen Laboratories, Cambridge, Mass.). Samples were assayed in duplicate, and IL-6 concentrations were derived from a standard curve comprised of serial dilutions (4.1–400 pg/ml) of purified recombinant human IL-6. Assay sensitivity was <1 pg/ml. The mean inter- and intraassay coefficients of variation were 10.9% and 3.6%, respectively. A subset of study participants (nine of the 10 healthy comparison subjects, 11 of the 12 depressed subjects without cancer, eight of the 13 cancer patients without major depression, and all eight of the cancer patients with comorbid major depression) also underwent a dexamethasone suppression test. The subjects ingested 1 mg of dexamethasone at 11:00 p.m., and blood samples were obtained at 4:00 p.m. the next day. A subject exhibited nonsuppression of cortisol, i.e., had an abnormal dexamethasone suppression test result, if the 4:00 p.m. plasma cortisol concentration was ≥5 ng/ml
(31–
34). Plasma concentrations of cortisol were measured by using radioimmunoassay (GammaCoat, Incstar [now DiaSorin], Stillwater, Minn.). The mean inter- and intraassay coefficients of variation were 8.8% and 6.6%, respectively. All biological samples were assayed by personnel who were blind to the diagnostic identity of the study subjects.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed to determine group differences in age. Results for age were presented as means and standard deviations. Since plasma concentrations of IL-6 and postdexamethasone plasma cortisol concentrations were not normally distributed within the groups, a Kruskal-Wallis one-way ANOVA on ranks was utilized to determine group differences on these two indices. The median IL-6 plasma concentrations and the 95% confidence interval (CI) of the median were also calculated within each group
(35). Fisher’s exact test was used to determine between-group differences in postdexamethasone plasma cortisol concentrations. Spearman rank-order correlations were used to determine the relationships among Hamilton depression scale scores, plasma IL-6 concentrations, and postdexamethasone plasma cortisol concentrations.
Results
Demographic and clinical characteristics of the study sample are presented in
Table 1. The study groups did not differ significantly in mean age (F=2.76, df=3, 39, p=0.055). However, the cancer patients with comorbid major depression were the eldest group, and the healthy comparison subjects were the youngest group. Hamilton depression scale scores indicating moderate severity of depression were observed in the two study groups with major depression, although the cancer patients without depression exhibited some depressive symptoms.
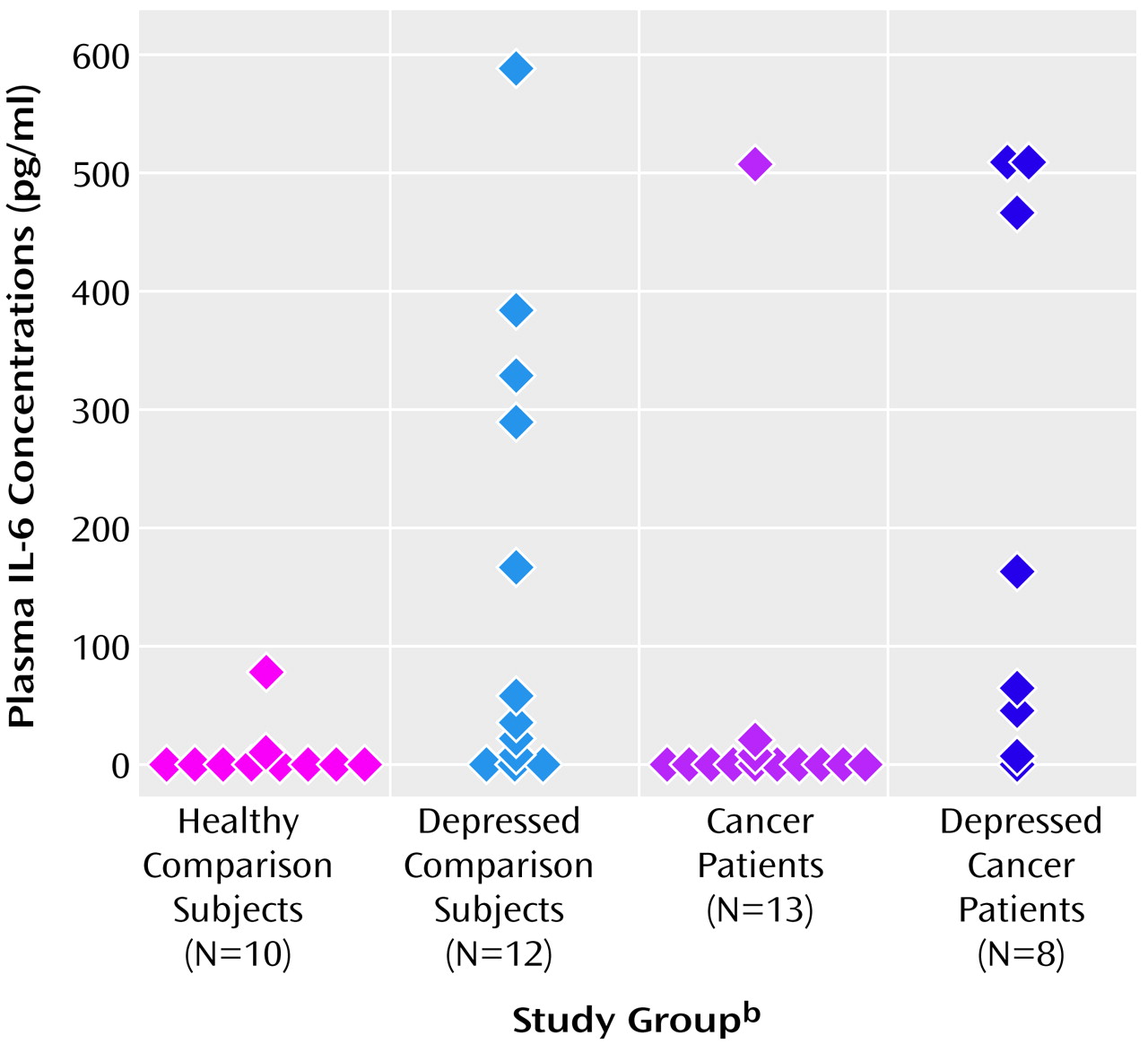
There was a significant difference in mean plasma concentrations of IL-6 between the four groups (
Figure 1). Among the four study groups, the cancer patients with major depression had the highest median plasma concentration of IL-6 (median=116.4 pg/ml, 95% CI=8.6–512.5), and the cancer patients without major depression had the lowest (median=0 pg/ml, 95% CI=0.0–0.0). The median plasma IL-6 concentrations for the depressed comparison group and the healthy comparison group were 50.0 pg/ml (95% CI=11.1–296.0) and 0.1 pg/ml (95% CI=0–2.2), respectively (
Figure 1).
Although there were no significant differences in mean plasma cortisol concentrations after dexamethasone administration among the four groups (H=4.7, df=3, p=0.20), analysis of postdexamethasone cortisol suppressor status revealed a significant overall difference among the four groups (p=0.02, Fisher’s exact test). Cancer patients with major depression exhibited the highest rate of postdexamethasone cortisol nonsuppression, compared to the other study groups. Of the eight cancer patients with major depression, five (63%) were nonsuppressors of cortisol (plasma cortisol concentrations ≥5 ng/ml), compared to one (13%) of the eight of the cancer patients without major depression. Moreover, three (27%) of the 11 subjects with major depression without cancer were cortisol nonsuppressors, compared to none of the healthy comparison subjects.
The relationships among Hamilton depression scale scores, postdexamethasone cortisol plasma concentrations, and plasma IL-6 concentrations were also explored. There was a positive correlation between Hamilton depression scale scores and postdexamethasone cortisol plasma concentrations (rs=0.35, df=30, p=0.05). The correlation between the Hamilton depression scale scores and postdexamethasone cortisol plasma concentrations was also evaluated within two subgroups: the depressed subjects (including subjects with depression alone and cancer patients with major depression) and the nondepressed subjects (including normal comparison subjects and cancer patients without major depression). The correlations were not significant (depressed: rs=0.36, df=15, p=0.16; nondepressed: rs=0.33, df=13, p=0.23). There were no significant correlations between Hamilton depression scale scores and plasma IL-6 concentrations (rs=0.24, df=37, p=0.14) or between plasma IL-6 concentrations and postdexamethasone cortisol concentrations (rs=–0.10, df=34, p=0.55).
Discussion
In this preliminary study of 21 patients with cancer, cancer patients with depression exhibited significantly higher plasma concentrations of IL-6, compared to cancer patents without depression and healthy comparison subjects. These higher levels of IL-6 were similar to levels observed in depressed subjects without cancer and are consistent with reports in the literature describing higher than normal IL-6 plasma concentrations in patients with major depression
(18,
36–
39). Indeed, proinflammatory cytokines such as IL-6 have the capacity to induce a syndrome of “sickness-behavior” that shares many features with major depression, including anhedonia, fatigue, anorexia, reduced activity, and altered sleep patterns
(14,
15). IL-6 is also an important mediator of the acute phase response, and higher than normal levels of acute phase proteins have been reported in depressed patients by our group and others
(18,
19). Nevertheless, the high levels of IL-6 in cancer patients with depression may reflect an epiphenomenon of the cancer disease process, rather than play a primary causal role in the pathophysiology of major depression in patients with cancer. However, if IL-6 plays a direct role in inducing major depression, cancer patients may be especially vulnerable to the behavioral and neuroendocrine consequences of IL-6 and other proinflammatory cytokines, including IL-1 and TNF, by virtue of immune activation secondary to tissue destruction and the associated inflammation. Such a scenario would suggest that patients with medical disorders (and associated inflammation) other than cancer also may be at risk for depression by virtue of increased peripheral release of proinflammatory cytokines, which in turn have central nervous system effects.
A major pathway by which IL-6 and other proinflammatory cytokines are regulated is the HPA axis. Glucocorticoids, the final product of HPA-axis activation, are potent inhibitors of cytokine activity and inflammatory responses
(40). Besides increased release, another mechanism that may contribute to higher than normal IL-6 in medically ill patients with depression is disruption of glucocorticoid-mediated feedback inhibition of cytokine production. Disruption of glucocorticoid-mediated feedback pathways is evidenced by postdexamethasone nonsuppression of cortisol. In this study, subjects with cancer and major depression exhibited a high rate of postdexamethasone cortisol nonsuppression (63%). In addition, the positive correlation between Hamilton depression scale scores and postdexamethasone cortisol plasma concentrations confirms and extends a large body of literature documenting the relationship between severity of major depression and alterations in glucocorticoid-mediated feedback inhibition
(41). Nevertheless, patients with certain types of cancers, i.e., cancers of the pancreas and/or gastroesophageal tract, may be more prone to postdexamethasone cortisol nonsuppression, irrespective of depression. For example, Joffe and colleagues
(7) reported that all of six patients with pancreatic cancer were cortisol nonsuppressors, even though only one of the six had a diagnosis of major depression. Moreover, five of six patients with gastric cancer were cortisol nonsuppressors, even though none of the gastric cancer patients was diagnosed with major depression. In this study, all of the four patients with pancreatic cancer and comorbid depression were cortisol nonsuppressors. However, only one of the four patients with pancreatic cancer without comorbid depression were cortisol nonsuppressors. Our results are consistent with those of Evans et al.
(42), who found that 11% of women with gynecologic cancers without psychiatric diagnoses exhibited postdexamethasone cortisol nonsuppression, compared to 40% of those with comorbid major depression. It should be noted that proinflammatory cytokines themselves can disrupt glucocorticoid-mediated negative feedback pathways through direct effects on the glucocorticoid receptor
(43). Although a relationship between IL-6 and postdexamethasone cortisol concentrations in depressed patients has been reported
(44), no correlation was found between these two variables in this study. The small size of the study groups and wide variations in plasma cytokine concentrations may have contributed to these results. Nevertheless, there may be a subpopulation of depressed patients with elevated IL-6 who exhibit postdexamethasone nonsuppression of cortisol.
The psychological distress and dysphoria observed in some patients with cancer represent complex interactions of social, psychological, and biological factors. The data presented herein indicate that one potential source of this distress may be IL-6, which is capable of exerting direct effects on CNS function leading to behavioral symptoms consistent with those seen in major depression. Future studies are warranted to elucidate the role of IL-6 in mood disorder in patients with cancer and/or other medical illnesses with associated immune activation.