An increasing number of clinical and epidemiology studies have implicated depression as a potential risk factor in the morbidity and mortality of a wide range of human diseases. Although neurobiological mechanisms remain unknown, there is a considerable body of basic and clinical research documenting a relationship between depression and cell-mediated immunity. If this relationship is clinically relevant, depression could alter key parameters of cellular immunity, thereby accelerating the course of an immune-based disease. Thus, we and others have investigated the relationship between depression and immune function in individuals with HIV infection.
The increasing spread of HIV to women has become a significant public health issue, and HIV is among the leading causes of death for U.S. women between the ages of 25–44
(1). However, there is a relative paucity of controlled data regarding the prevalence of depression in HIV-seropositive women. Although estimates of depression vary widely among the available studies of HIV-seropositive women, the prevalence of depression appears to be at least twice as high in women with HIV infection compared with HIV-seropositive men
(1,
2). If depression is associated with greater HIV morbidity and mortality, women could be particularly vulnerable, given this high prevalence of depression. In fact, a recent, large epidemiology study found a significant association between depression and HIV morbidity and mortality in HIV-seropositive women. The mortality rate was doubled in the women with chronic depressive symptoms compared with women with limited or no depressive symptoms
(2).
Given the wide variability in the progression of HIV infection, many studies have examined the impact of depression on the course of HIV infection. Recent studies of HIV-seropositive individuals have demonstrated an association between depression and both early and late HIV disease progression
(3–
5) as well as mortality
(2). There is considerable evidence from meta-analyses
(6) as well as from studies by our group and others
(7–
10) that measures of cellular immunity are altered in depressed subjects without medical illness. However, in depressed HIV-seropositive individuals, findings have been mixed
(1), although several studies have found a significant relationship between depression and immune system measures
(11–
14). These inconsistent findings may be partially attributed to methodological differences among the available studies, including in their measurement of depression, duration of follow up, differences in the immune measures examined, and differences in the subject populations studied.
The potential immune mechanisms by which depression may influence HIV disease progression and mortality remain to be understood. Most studies assessing the effects of depression on immunity in HIV infection have focused primarily on CD4 cell populations and have used short periods of observation. Because CD4 cells are affected early and profoundly in HIV infection, this cell population may not be the most sensitive or reliable measure for demonstrating over time the relationship between depression and HIV infection
(8,
11).
Clinical studies of depression in subjects without other medical illness have demonstrated significant alterations in natural killer (NK) cells as well as CD8 cells, two cellular immune populations that may play key roles in regulating HIV infection. Specifically, NK cells may be involved in natural resistance against viral infection and may have the capacity to lyse HIV-1 infected cells
(15–
18), while subsets of CD8 cytolytic cells may inhibit HIV-1 replication in early stage HIV disease
(19–
23). In our studies of HIV-infected men, we have found depression-associated alterations of NK cells and CD8 T lymphocytes
(12), which suggests that killer lymphocytes might mediate the effects of depression on earlier stages of HIV disease progression. CD8 T lymphocytes may have a beneficial or adverse effect in later stage infection
(24–
28). Most recently we have found CD8 T lymphocytes are elevated in HIV-infected men with advanced HIV disease (unpublished 2001 study of D. Evans et al.). A subpopulation of activated CD8 cells (CD8+/CD38+/DR+ activated CD8 cells) have been correlated with cytotoxic activity and with HIV disease progression
(20,
29,
30).
The purpose of the present clinical study was to determine if depression was associated with alterations in cellular immunity in women with HIV infection. We specifically focused on killer lymphocytes (NK cells and CD8 T lymphocytes) on the basis of the extensive immunologic literature suggesting a potential role for these lymphocytes in the host defense against HIV and also on the large psychiatric literature suggesting that depression is associated with significant alterations in these lymphocyte populations. We used flow cytometry with selected monoclonal antibodies in order to identify subpopulations of NK and cytotoxic T cells, including activation markers that have been associated with HIV disease progression
(29). Although NK and CD8 T lymphocyte populations have been studied in HIV-infected men as possible mechanisms linking depression to HIV disease progression
(11,
12), no previous study to our knowledge has examined these possible cellular-immune mechanisms in HIV-infected women, despite the fact that depression is approximately two times more prevalent in women than in men.
Method
Data were collected in Florida and Pennsylvania as part of an ongoing longitudinal cohort study investigating neuropsychiatric, psychosocial, neuroendocrine, and immune aspects of HIV infection in women. Data from the baseline visit were used for this analysis.
Subjects
HIV-seropositive subjects were recruited from outpatient medical clinics, county health departments, and organizations focusing on HIV illness and care through a combination of community outreach presentations, clinician referrals, word of mouth, and newspaper advertisements to identify potential subjects. The seronegative subjects were recruited by word of mouth, advertisements, and by inviting enrolled subjects to recruit a friend or neighbor. Subjects were included in the study if they were female, between 18 and 70 years of age, and able to communicate in English. HIV serostatus was determined by enzyme-linked immunoabsorbent assay and confirmed by Western blot analysis. Subjects were excluded if they 1) had significant chronic, systemic illness; 2) had a significant neurologic disorder, including traumatic brain injury; 3) had a history of schizophrenia or severe psychotic disorder; 4) were pregnant or nursing; or 5) met DSM-IV criteria for current substance/alcohol abuse or dependence.
The protocol was reviewed and approved by the institutional review boards of both the University of Florida and the University of Pennsylvania. All subjects provided written informed consent and were reimbursed for their time, travel expenses, and child care.
Procedures
Each subject received a thorough outpatient assessment that included a physical examination and a structured psychiatric interview. Subjects also completed a comprehensive set of questionnaires that assessed mood, psychosocial factors, and health habits. Current and lifetime DSM-IV axis I diagnoses were assessed by a research psychiatric clinician with a modified Structured Clinical Interview for DSM-III-R
(31,
32). Consensus diagnoses were determined at diagnostic conferences. Symptoms of depression and anxiety were evaluated with the 17-item Hamilton Depression Rating Scale
(33) and the Hamilton Anxiety Rating Scale
(34). We also used a modified version of the Hamilton depression scale that eliminated six of the 17 symptom items (e.g., somatic symptoms, weight loss, retardation) that could overlap with the physical symptoms of HIV disease
(12) to help avoid confounding depression with HIV disease.
All of the HIV-seropositive women were aware of their HIV-1 status at baseline. HIV serostatus for all subjects was confirmed by using enzyme-linked immunosorbent assay with Western blot analysis for confirmation of the presence of anti-HIV-1 antibodies.
To control for potential circadian effects on immunity, all subjects were studied at the same time of day, as in our previous studies
(10). Specifically, subjects were placed in a recumbent position, an intravenous line was started at approximately 9:00 a.m., and intravenous line patency was maintained with a slow, normal saline drip. Blood was drawn approximately 1 hour later
(35).
Immune System Measures
Complete blood cell counts and flow cytometry panels were performed on peripheral blood samples from all subjects. For the flow cytometry panel, the specimens were collected in EDTA tubes with a minimum of 2 μl of blood and the specimens were processed within 30 hours from the time of collection. Leukocyte viability was greater than 97%, and temperature variability during shipment was less than 2°C. Monoclonal antibodies (Becton Dickinson Immunocytometry System, San Jose, Calif.) were used to measure the predominant reactivity to the following lymphocyte subsets: CD3+/CD4+; CD3+/CD8+; CD3–/CD56+/CD16+ (NK cells); and CD8+/CD38+/DR+ (activated CD8+)
(36). All studies were performed by a dual- or tri-labeling (double- or triple-staining) technique, with the combination of fluorescein isothiocyanate and phycoerythrin-conjugated antibodies. Absolute lymphocyte subset counts were derived from lymphocyte subset percentages × total lymphocyte counts × 100.
NK cell activity was assessed by using a modification of standard techniques that we have established
(37). Aliquots of 100 microliters of various CD4+, NK cells, and function concentrations of peripheral blood mononuclear cells (effector cells) were incubated in triplicate with 100 microliters of labeled target cells for 4 hours at 37°C with 5% CO
2 (effector/target ratios of 50:1, 25:1, 12.5:1, and 6.25:1 were studied). Maximum release of
51Cr was determined by addition of 100 microliters of 1% Triton X solution to triplicate wells of K562 cells. At the end of the incubation period, 100 ml of the supernatants were harvested and counted in a gamma counter (Beckman Instruments, Inc., Fullerton, Calif.). NK activity was calculated as ([mean sample activity – mean spontaneous release]/[mean maximum release – mean spontaneous release]) × 100. The results were expressed as percent cytotoxicity.
Lytic units/10
7 peripheral blood mononuclear cells and lytic units/10
7 NK cells were calculated after the method of Bryant et al.
(38) and Friberg et al.
(39) in order to transform cell lysis data into a single number. Further, by measuring the percentage of CD16+/CD56+ cells in the peripheral blood mononuclear cell preparation, we determined the lytic units of NK activity per NK cell
(40). Expressing the NK data as lytic units per NK cell adjusts for the differences in the percentage of NK cells (CD16+/CD56+) in the effector cell population (peripheral blood mononuclear cells).
Statistical Analyses
Two sets of statistical analyses were performed: 1) baseline comparisons between HIV-seropositive and HIV-seronegative participants with respect to immune and depression/anxiety outcomes and 2) separate analyses of correlations between immune and depression/anxiety outcomes separately for HIV-seropositive and HIV-seronegative participants. For the baseline comparisons of the HIV-seropositive and seronegative participants, we used nonparametric tests of group differences (i.e., Kruskal-Wallis test). We relied on nonparametric statistics instead of transforming outcomes in an attempt to achieve normality. The results are similar between analyses of transformed data and nonparametric analyses. We also used the nonparametric Kruskal-Wallis test to compare the HIV-seropositive and HIV-seronegative women with respect to the 11-item and 17-item Hamilton depression scale scores and anxiety variables and used Fisher’s exact test to compare the rate of major depression in the two groups. For the correlational analyses, Spearman correlations were computed after we adjusted for antiretroviral medication use, and viral load was dichotomized by the measurement threshold level in the HIV-seropositive group. Since approximately one-half of the HIV-seropositive subjects had a viral load at 400 RNA copies/ml, we dichotomized the viral load variable at 400 (i.e., <400 RNA copies/ml and ≥400 RNA copies/ml) for analysis because there is no transformation that would resolve the skewed distribution problem at 400. We used the nonparametric Spearman approach because transformations, such as the log or square root transformation, did not successfully transform distributions to normality such that the assumptions underlying the Pearson correlation approach could be satisfied
(41).
To adjust for disease status, we controlled for viral load and antiretroviral medication use. Serum HIV RNA viral load was determined from archived samples with the Amplicor Monitor assay (Roche Diagnostics, Branchburg, N.J.). The lower limit of quantification for this assay is 400 copies/ml blood. Because approximately half the sample was at this lower limit, we analyzed viral load as a binary variable: equal to 400 and greater than 400.
Results
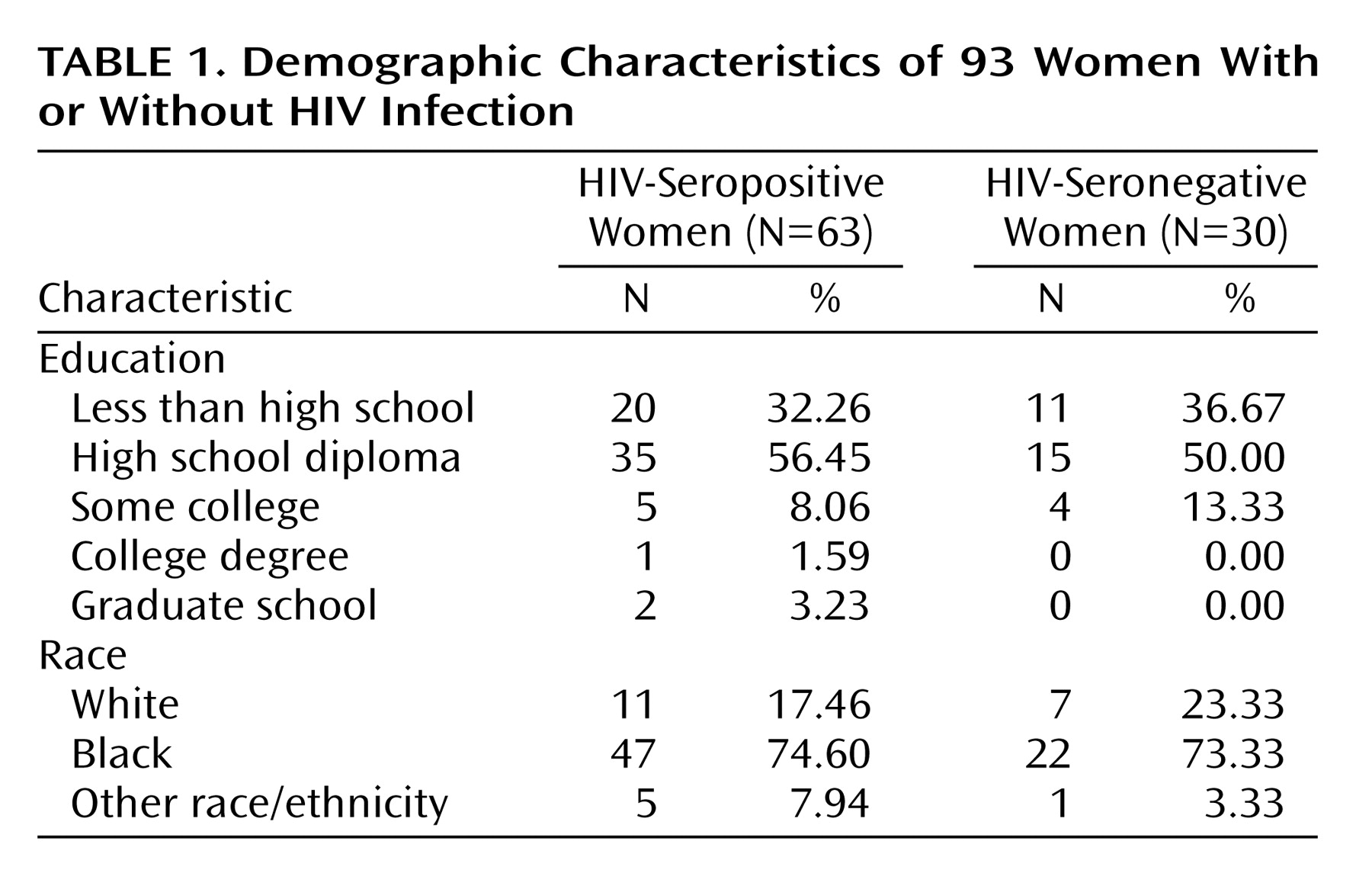
A total of 93 women were studied at two sites, 49 (52.6%) from Pennsylvania (35 HIV-seropositive and 14 HIV-seronegative) and 44 (47.3%) from Florida (28 HIV-seropositive and 16 HIV-seronegative). The demographic and behavioral characteristics of the two sites were similar. The racial composition was predominantly African American at both sites (Pennsylvania: 73.4%, N=36; Florida: 75.0%, N=33). Although Pennsylvania seropositive subjects had somewhat more education than did the Florida seropositive subjects (high school diploma: 62.9% [N=22] versus 46.4% [N=13], respectively), the difference in education did not reach statistical significance (χ
2=1.34, df=1, p=0.06). Depressive and anxiety symptom scores (from the 11- and 17-item Hamilton depression scale and the Hamilton anxiety scale) also were similar across the sites. Comparable 17-item Hamilton depression scale scores between Pennsylvania and Florida subjects were seen for the HIV-seropositive subjects (mean=8.60, SD=5.37 [N=35] and mean=8.64, SD=9.21 [N=28], respectively) and the HIV-seronegative women (mean=5.36, SD=5.37 [N=14] and mean=5.94, SD=8.87 [N=16]). Overall, the median age of the HIV-seropositive subjects was 38 years (range=19 to 60), and the median age of the seronegative subjects was 40 years (range=18 to 69).
Table 1 displays demographic characteristics of the study group.
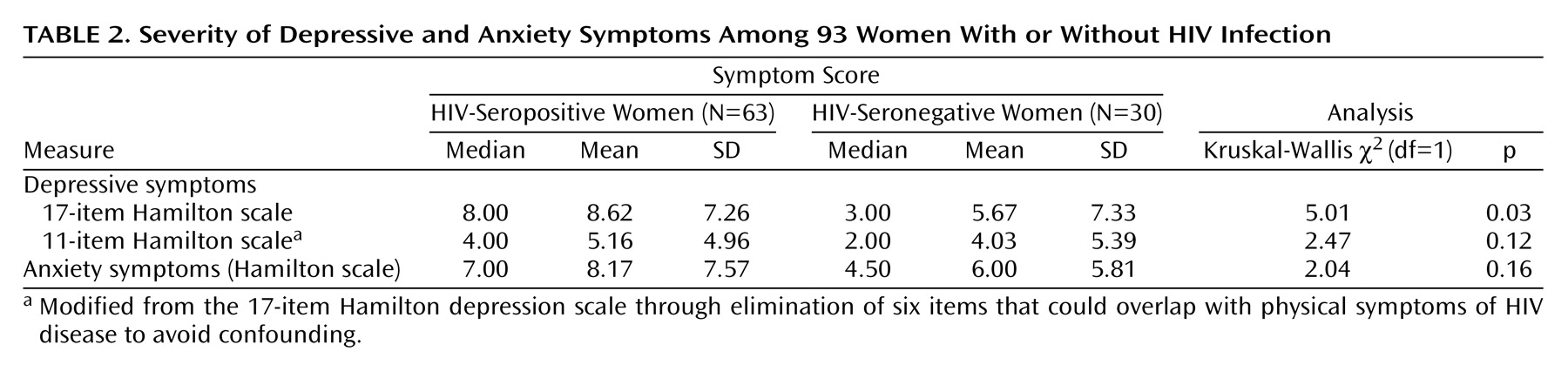
Table 2 presents the depression and anxiety characteristics of the HIV-seropositive and seronegative subjects. The rate of current major depression in the HIV-seropositive subjects was 15.87% (N=10 of 63), which was not significantly higher than the rate seen in the seronegative women (10.00%, N=3 of 30) (p<0.54, Fisher’s exact test). The HIV-seropositive subjects had a higher level of depressive symptoms than did the HIV-seronegative subjects, as measured by the 17-item Hamilton depression scale. Both serostatus groups had similar levels of anxiety symptoms as measured by the Hamilton anxiety scale.
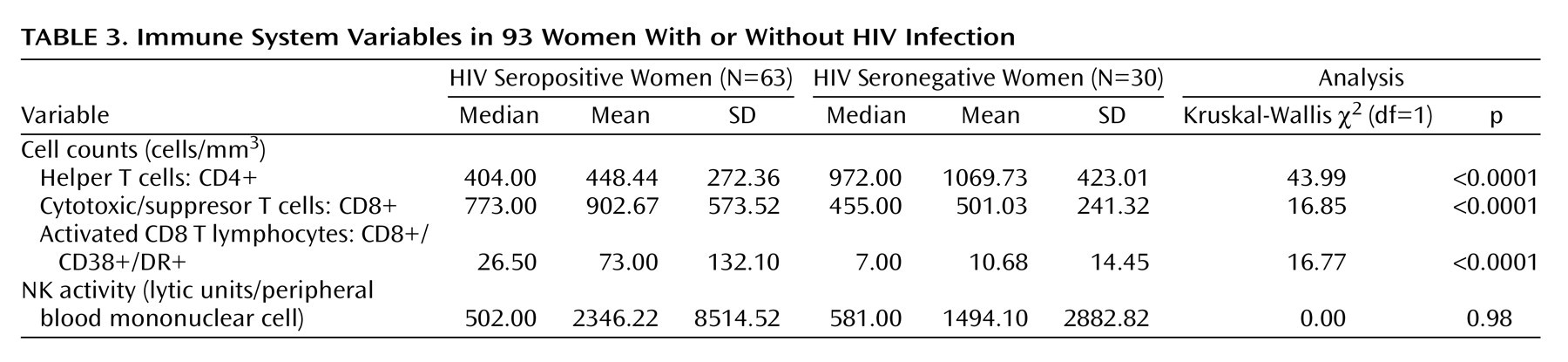
Differences in immune system variables between the HIV-seropositive and HIV-seronegative subjects are shown in
Table 3. Consistent with HIV infection, the HIV-seropositive women had significantly lower CD4+ cell counts and significantly higher CD8+ and activated CD8+ cell counts. There were no significant differences in natural killer cell activity (expressed as lytic units/peripheral blood mononuclear cells) between HIV-seropositive and seronegative groups.
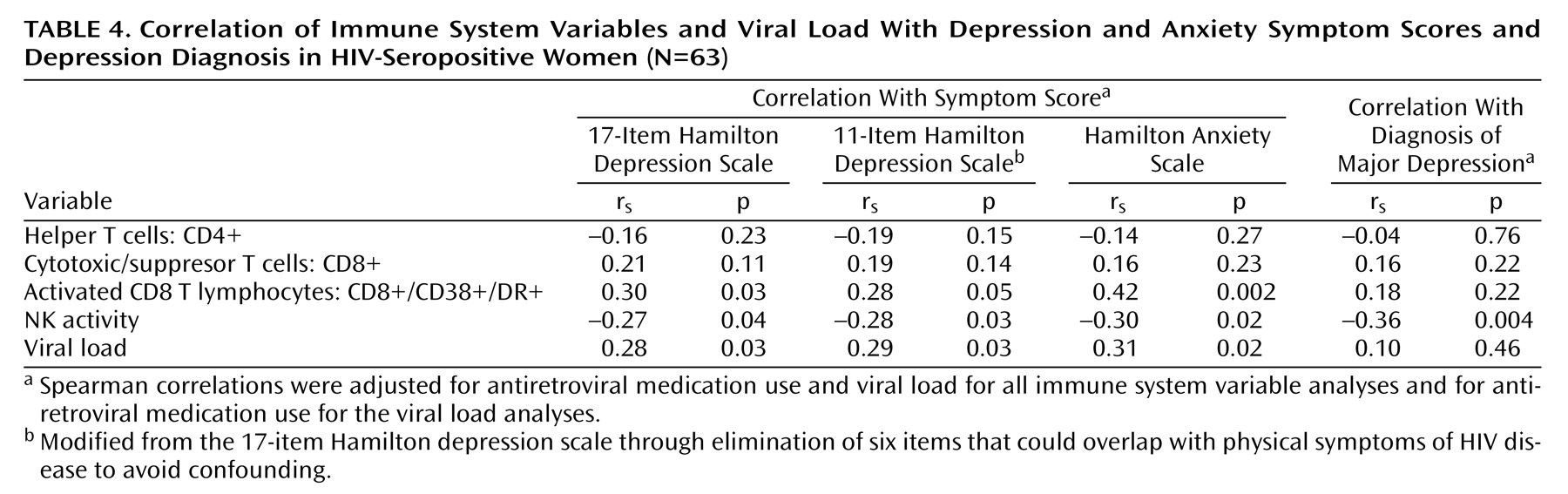
The relationship of a major depression diagnosis as well as depressive and anxiety symptoms to the immune system variables in HIV-seropositive women are presented in
Table 4. These correlations were adjusted for antiretroviral medication and viral load.
Depressive symptoms, as measured by the 17-item Hamilton depression scale, were significantly associated with higher activated CD8 T lymphocyte (CD8+/CD38+/DR+) counts and viral load levels. The 11-item Hamilton depression scale, which removes possible confounding physical symptoms, had essentially the same associations as the 17-item scale. Anxiety symptoms were significantly related to higher activated CD8 T lymphocyte counts and viral load.
Major depression, which occurred in 15.87% of the HIV-seropositive women (N=10 of 63), was associated with significantly lower natural killer cell activity. Depressive and anxiety symptoms showed similar correlations with lower natural killer cell activity. We found no relationship for CD4 cell count, CD8 cell count, or CD56+ cell count with depressive symptoms, anxiety symptoms, or diagnosis of major depression. Among the HIV-seronegative women, neither depression nor anxiety was related to any of the immune system variables (p>0.10). However, the 11-item Hamilton depression scale and 17-item Hamilton depression scale correlations with natural killer cell activity ranged between –0.20 and –0.30, values which are of similar magnitude and in the same direction as the analogous correlations in the HIV-seropositive subjects. The lack of significance may be due to the relatively small size of the HIV-seronegative group or greater immune system sensitivity to depression in individuals with HIV infection.
Discussion
This study is the first systematic report to show that depression is related to alterations in killer lymphocytes and viral load in women with HIV infection. These data demonstrate that depression and anxiety are associated with alterations in these measures of immunity in HIV-seropositive women. Specifically, we found that women with major depression exhibited significantly lower NK cell activity. In addition, depressive symptoms and anxiety symptoms were associated with lower NK cell activity and higher activated CD8 T lymphocyte levels and viral load. Since each of these measures has been associated with disease progression in HIV, together these findings suggest that depression may be associated with a higher likelihood of disease progression.
The findings from the present study of HIV-infected women extend our previous investigations in HIV-infected men by showing significant killer lymphocyte alterations. In this study of women, we have expanded our assessment of the cellular-immune system by including a functional measure (lytic units per NK cell), which measures natural killer cell activity adjusted for the number of NK cells in the assay performed. Our finding of lower natural killer cell activity in association with depression could be clinically relevant, since NK cells have the capacity to lyse HIV-infected cells and may be involved in the host defense against viral infection
(15–
18,
42).
Our finding in HIV-seropositive women that depression is associated with significant increases in subsets of CD8 cells that represent activated CD8 T lymphocytes is a new finding. Previously, we reported significant decreases in CD8 cells in association with depression and stress in HIV-seropositive men
(12). These findings appear consistent with recent evidence suggesting that the CD8 T lymphocytes may play a beneficial role in early HIV disease infection and may, in fact, be detrimental to the host in the defense against HIV later in the course of disease
(24). In early HIV disease, there is evidence that populations of CD8 T lymphocytes expand in what is believed to be a compensatory immune response to control HIV infection by inhibiting viral replication and by lysing HIV-infected cells
(43–
45). However, recent evidence suggests that in HIV-infected individuals with progressive disease, CD8 T lymphocyte responses could have a deleterious effect on the immune system and thereby have a negative effect on HIV disease progression
(24). In fact, studies assessing CD8 cells with activation markers (CD38+, HLA-DR) as used in the present study have found strong associations with greater viral load, lower CD4 count, progression to AIDS, and mortality
(29,
30). Thus, depression-associated increases in the subsets of activated CD8 T lymphocytes as found in the present study also may be a mechanism by which depression may have a negative effect on HIV disease survival
(2).
In the present study, we found no relationship between depression and CD4 cells, which is consistent with several previous studies
(46–
48). These previous studies did not assess NK cells or CD8 T cell subsets. Further, NK cells are among the major cells of the innate immune system. NK cells are capable of eliminating HIV-infected cells both by direct cytotoxicity and by antibody-directed cytotoxicity. NK cells produce cytokines and chemokines
(28) that also are important in HIV responses.
There is increasing evidence that depression may be predictive of human morbidity and mortality in a wide range of diseases
(49–
53). Studies of depressed but otherwise medically healthy individuals suggest that depression may alter cellular immunity and may therefore contribute to disease progression in certain immune diseases such as HIV infection
(1). Thus, the present study suggests that depression-related alterations in NK and CD8 cells in HIV-seropositive women could be one of the mechanisms underlying the association between depression and mortality in the recent epidemiology study of HIV-seropositive women
(2). Since viral loads were also higher, another consideration includes the role of adherence to antiretroviral medication treatment. Poor adherence has been reported in depressed individuals
(54) and in depressed HIV-seropositive individuals
(55–
58). In the present study, we controlled for the potential confounding effects of lack of adherence by controlling for two surrogate markers of adherence (reported medication use and viral load) in all depression-immune analyses. In future studies, we plan to use electronic monitoring systems as an additional surrogate marker of adherence in order to assess the direct effects of depression on HIV medication adherence
(56,
59,
60).
Several strengths of the present study should be noted. Subjects underwent comprehensive structured interviews to assess psychiatric diagnoses as well as mood and anxiety symptoms. In order to address carefully the effects of depression on cellular immune function, we excluded subjects with current alcohol or substance abuse or dependence to avoid the potential confounding effects on immune function. We focused on specific lymphocyte subsets that are believed to play an important role in host resistance against HIV infection, and we standardized our biological assessments by performing phlebotomy at the same time of day and following 1 hour of rest in a recumbent position to avoid potential circadian effects on immunity and potential nonspecific methodological factors
(11,
12,
35). In all depression-immune analyses, we controlled for stage of disease by controlling for viral load as well as antiretroviral medication use. We also performed depression analyses with the traditional 17-item Hamilton depression scale, as well as an 11-item instrument that eliminated those physical symptoms that might possibly overlap with symptoms of HIV disease progression.
Some possible limitations of this study should be noted. This was not a population-based study, and thus the results may be biased by the methods of recruitment and enrollment. For example, recruiting comparison subjects through friendship with participants could bias toward better mental health, since individuals with someone to refer may have a larger or more active social support network from which to recruit. Although recruitment was open to subjects of any race and ethnic background, African American and Caucasian subjects comprised the majority of the subjects. A small number of subjects of “other ethnicity” were enrolled; however, the findings may not be generalizable to Hispanic or Asian populations. The study eligibility criteria excluded women who were currently abusing alcohol or other substances. This exclusion avoided confounding our results with the known association of substance/alcohol abuse and depression, but it limits the generalizability of our findings. Subjects were recruited from two sites, one in Florida and one in Pennsylvania. Before the data were analyzed, comparisons of the site-specific demographic characteristics and behavioral characteristics of subjects did not reveal any substantive differences between the sites in demographic or behavioral characteristics. All psychiatric diagnoses were confirmed at consensus conferences, and interviewers as well as consensus reviewers were not blinded regarding the serostatus of study participants. In our previous studies, we have not found a satisfactory way to maintain such a blind and achieve validation of the consensus diagnosis. Therefore, there is a small but potential bias in psychiatric diagnoses.
The finding that 16% of HIV-seropositive women without current substance abuse have current major depression represents a prevalence rate that is approximately twice that reported for HIV-seropositive men
(32,
61–63). Of note, this rate of current major depression is similar to that found in studies of women with other medical illnesses, such as cancer
(64) and heart disease
(65). It is also consistent with a recent epidemiology study associating depression to mortality in HIV-infected women in which 42% of seropositive women experienced chronic depressive symptoms when depressive symptoms were measured by the CES-D Scale, a self-report screening instrument
(2).
In conclusion, our findings provide the first evidence that depression may alter the function of killer lymphocytes in HIV-seropositive women. These findings suggest that depression may decrease natural killer cell activity and may lead to an increase in activated CD8 T lymphocytes and viral load. Increasing evidence suggests that depression may have a negative impact on innate immunity, and the present study suggests that the examination of killer lymphocytes may prove useful in assessing the potential relationship between depression, immunity, and HIV disease progression in women.