Current neurobiological models of obsessive-compulsive disorder (OCD) emphasize frontal-striatal system dysfunction
(1). The striatum supports some aspects of “implicit learning,” which refers to the process by which knowledge is acquired by means of repetition or exposure and expressed without conscious reference to the learning episode. In contrast, “explicit learning” refers to memory functions requiring conscious encoding or retrieval of facts and relies on the integrity of the frontal and medial temporal lobes
(2).
A well-characterized measure of implicit learning is the serial reaction-time task
(3). In this task, participants are presented with sequences of visual cues in which the locations of the cues are predictable or unpredictable. Implicit learning is demonstrated by the reaction-time advantage in response to predictable versus unpredictable sequences after the subject has been exposed to repeated trials of the predictable sequences.
In positron emission tomography and functional magnetic resonance imaging studies
(4,
5), OCD patients have exhibited normal implicit reaction-time task learning. However, whereas healthy subjects showed activation in the right striatum when the predictable versus the unpredictable conditions were contrasted, the OCD patients failed to show right striatal recruitment. Instead, they exhibited activation in medial temporal regions (i.e., the hippocampal/parahippocampal cortex)—brain regions normally involved in explicit memory. This abnormal pattern of activation along with normal behavioral performance on the reaction-time task suggests that OCD patients may use medial temporal networks to compensate for striatal dysfunction
(4).
The current study was designed to test this hypothesis of compensatory neural processing in OCD by using a dual-task reaction-time task that entails a concurrent explicit memory load in addition to the reaction-time task-sequence learning task. We predicted that a simultaneous explicit memory load might disproportionately interfere with implicit sequence learning in OCD patients if it prevents the compensatory use of brain systems normally involved in processing explicit information.
Method
Study participants were 25 right-handed subjects who met DSM-IV criteria for OCD (19 women) and 25 right-handed healthy comparison subjects (15 women) who were recruited through Massachusetts General Hospital’s OCD unit. All participants rendered written informed consent before participation, in accordance with Massachusetts General Hospital’s Subcommittee on Human Studies. Current comorbid (secondary) diagnoses in OCD subjects included major depression (N=5), dysthymia (N=2), body dysmorphic disorder (N=2), panic disorder (N=3), social phobia (N=3), tic disorder not otherwise specified (N=1), and anorexia nervosa (N=1). Fifteen OCD subjects were taking psychotropic medication at the time of testing: selective serotonin reuptake inhibitors (N=9), benzodiazepines (N=5), risperidone (N=2), and phenelzine (N=1). In the OCD group, Yale-Brown Obsessive Compulsive Scale
(6) and Beck Depression Inventory scores indicated moderate OCD severity (mean=21.3, SD=5.8) and low levels of depression (mean=13.2, SD=9.0). All subjects were otherwise medically healthy by self-report and reported no history of significant head injury, seizure, neurological condition, or current major medical condition. OCD and comparison subjects did not differ with regard to age (t=–1.13, df=48, p=0.27) or education (t=–0.11, df=48, p=0.91).
For a detailed description of the version of the reaction-time task that we used, see Whalen et al.
(7). Briefly, in the dual-task reaction-time task, participants respond to visual cues on a screen by pressing one of four buttons that corresponds to the location of the cue. Each trial consists of a sequence of three such cues; the third cue in the sequence is either predictable or unpredictable on the basis of the location of the first two cues. Each participant completes three blocks of 36 trials (1×12 unpredictable sequences, and 3×8 predictable sequences). In addition, at the beginning of each block, subjects are given a seven-letter explicit working memory load (e.g., “GFMSVRJ”) and instructed to maintain the letters in working memory throughout the reaction-time task for a free-recall test after each block. Participants were instructed to give attentional priority to the working memory components of the dual-task reaction-time task.
After completion of the third block, subjects were tested for explicit knowledge of the cue sequences by using a frequency-recognition task in which subjects rated the frequency with which predictable and unpredictable sequences occurred in the third block.
Data were analyzed by using mixed-model analysis of variance (ANOVA) with group (OCD versus comparison) as the between factor, block and (for reaction time only) type of trial (predictable versus unpredictable sequences) as within factors, followed by unpaired and pairwise t tests. Dependent variables were 1) the mean reaction time to the third cue in predictable and unpredictable sequences, 2) the number of correctly recalled letters from the working memory task, and 3) the frequency rating for the recognition test.
Results
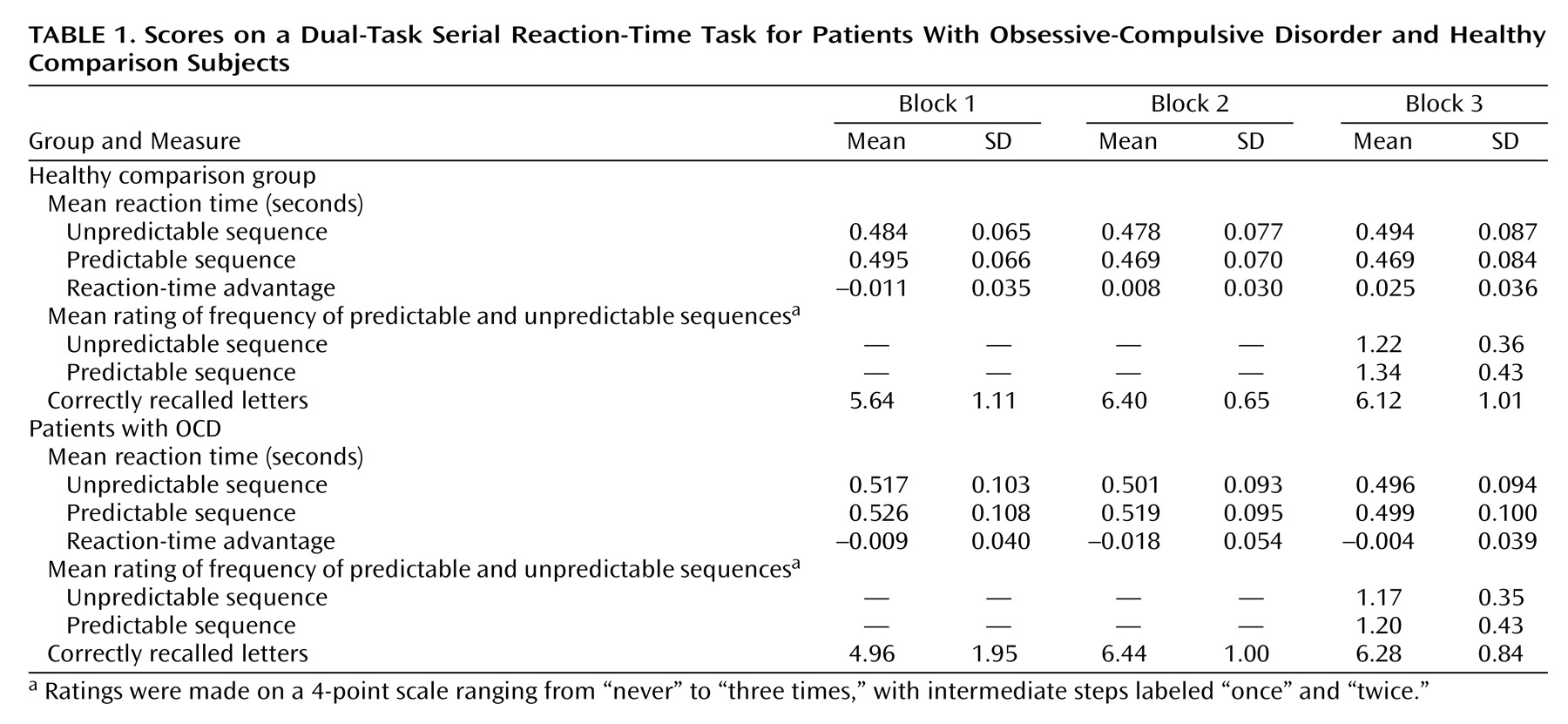
With regard to explicit working memory performance (recall for letters), ANOVA indicated a main effect for block (F=20.37, df=2, 96, p<0.001), a less-than-significant group-by-block interaction (F=2.99, df=2, 96, p<0.06), and no significant main effect for group (F=0.41, df=1, 48, p=0.53) (
Table 1). Follow-up unpaired t tests indicated that OCD and comparison subjects did not differ significantly in the number of letters recalled after the first block (t=1.52, df=48, p=0.14), the second block (t=–0.17, df=48, p=0.87), or the third block (t=–0.61, df=48, p=0.55) (
Table 1).
With regard to implicit sequence-learning performance (reaction time), ANOVA indicated a significant main effect for block (F=5.98, df=2, 92, p=0.004), a significant group-by-trial type interaction (F=6.06, df=1, 46, p<0.02), a significant interaction for block-by-trial type (F=4.54, df=2, 92, p<0.02), and a significant group-by-block-by-trial type interaction (F=3.12, df=2, 92, p<0.05). For OCD patients, follow-up pairwise t tests between reaction time and predictable and unpredictable trials within each group indicated no significant differences between predictable and unpredictable trials in the first block (t=–1.14, df=23, p=0.27), the second block (t=–1.67, df=24, p=0.11), or the third block (t=0.53, df=24, p=0.60). For comparison subjects, there was no difference in reaction time to predictable and unpredictable trials in the first block (t=–0.16, df=23, p=0.13) or the second block (t=1.36, df=24, p=0.19). However, there was a significant difference between predictable and unpredictable trials in the third block (t=3.47, df=24, p=0.002). This indicated a learning effect for predictable trials in the comparison subjects but not in the OCD subjects. Moreover, an unpaired t test between comparison and OCD subjects indicated a significantly greater reaction-time advantage (reaction time to unpredictable sequences minus reaction time to predictable sequences) for the comparison subjects than for the OCD subjects in the third block (t=2.73, df=48, p=0.009).
With regard to explicit knowledge for sequences (frequency ratings), ANOVA indicated no main effects for group (F=1.06, df=1, 48, p=0.31) or trial (F=1.44, df=1, 48, p=0.24) and no significant interaction (F=0.50, df=1, 48, p=0.48) (
Table 1). Both comparison and OCD subjects estimated that predictable and unpredictable triplets occurred on average only once, indicating that both groups were unaware of the predictable sequences.
With regard to medication and comorbidity, after exclusion of either medicated patients or patients with comorbid diagnoses, replication of the described analyses yielded results equivalent to those for the total OCD group in both the subgroup of medication-free patients as well as the subgroup of comorbidity-free patients.
Discussion
Consistent with our hypothesis, OCD subjects failed to exhibit implicit sequence learning in this dual-task reaction-time task. Specifically, only comparison subjects—not OCD patients—showed a reaction-time advantage for predictable sequences compared to unpredictable sequences in the third block. Moreover, comparison subjects developed a significantly greater reaction-time advantage than OCD patients, which is indicative of impaired sequence learning in OCD patients. OCD subjects exhibited no deficit in explicit information processing, as assessed by recall for letters. Furthermore, ratings about the frequency with which the various triplets had appeared in the third block of the experiment indicated that both OCD and comparison subjects had no significant awareness of the predictable sequences. This indicates that preserved sequence learning ability under the dual-task condition in comparison subjects did not reflect explicit knowledge as a basis for better reaction time. Taken together, the finding of impaired sequence learning in OCD subjects but preserved processing of explicit information and absent awareness of the repeated sequences suggests that concurrent processing of explicit information interfered with implicit learning of the sequences presented in the dual-task reaction-time task as originally hypothesized.
However, it is important to acknowledge that we did not assess implicit sequence learning using both the single- and dual-task reaction-time tasks for the same subjects. Consequently, our interpretation of the current findings relies in part on prior findings. In addition, one should be cautious about linking the current cognitive behavior results with interpretations regarding neurocircuitry from prior neuroimaging findings. The results of this study do not allow firm conclusions regarding the neural underpinnings of the parallel-processing deficit exhibited by subjects with OCD.
We originally hypothesized that OCD patients may employ medial temporal networks to learn sequence information as a means of compensating for primary dysfunction in frontal-striatal systems. From this perspective, parallel processing of explicit information may have “preoccupied” frontal-temporal systems, thereby preventing subjects with OCD from employing these structures for sequence learning and hence uncovering a limitation in implicit learning and memory. However, at this time, it cannot be ruled out that concurrent explicit information-processing demands interfere with implicit learning in patients with OCD by some other mechanism, nor does intact letter recall in OCD subjects preclude the possibility of dysfunction involving the medial temporal lobe. Future studies are needed to clarify the neural substrates of normal parallel-processing functions as well as their apparent disruption in OCD.