Schizophrenia is a serious chronic psychiatric disorder affecting approximately 1% of the population around the world and is usually characterized by a progressive decline in functioning, although its course may vary considerably
(1,
2). Progression in various aspects of the illness has been found, including an increase in the severity of negative symptoms
(3), overall cognitive decline as measured with the Clinical Dementia Rating Scale
(4), and a decrease in abstract thinking
(5). Other cognitive functions in patients showed changes that were comparable with those found in healthy comparison subjects
(5).
The etiology of schizophrenia is unknown, but numerous findings from imaging studies strongly support the view that schizophrenia is a brain disease particularly involving decrements in gray matter volume
(6–
9). It has not been resolved whether the structural brain changes are static or increase over the course of the illness. Progressive decrements in volumes of total brain matter
(10), the frontal lobe
(11), and frontal and superior temporal gray matter
(12) have been reported in patients with recent-onset
(10,
11) and chronic
(12) schizophrenia, with follow-up intervals of 2–5 years. In patients with childhood-onset schizophrenia, a fourfold decrease in cortical gray matter volume and an increase in ventricular volume were reported in a 2-year longitudinal study
(13,
14). Some studies have suggested an increase in ventricular volume in chronically ill patients over time
(12,
15–
18), although usually only in those with a poor outcome of the disease
(15–
17, but see reference
12) or in a subgroup of patients
(18). In other studies no changes in ventricular volume were found
(19–
23). Thus, the available evidence, although limited, suggests that progressive changes in brain volume may occur in schizophrenia patients
(24). However, it remains unresolved whether brain changes in patients with schizophrenia progress across the entire course of the illness. Although ultimately this question will have to be answered in a longitudinal study, changes measured cross-sectionally can provide suggestive evidence in this regard.
We compared brain volumes across a patient group with a 55-year age span by means of magnetic resonance brain images of 159 patients with schizophrenia and 158 healthy comparison subjects. Our aim was to determine whether age-related changes in brain volumes are excessive in schizophrenia patients.
Method
Subjects
A total of 159 patients (112 men, 47 women) with schizophrenia or schizophreniform disorder and 158 healthy comparison subjects (106 men, 52 women) from the Utrecht Schizophrenia Project participated after written informed consent was obtained. These subjects have been described
(25). All subjects were between 16 and 70 years of age. Subjects with a major medical or neurological illness, including past head trauma, hypertension, cardiac disease, diabetes mellitus, cerebrovascular disease, epilepsy, migraine, endocrine disorders, drug or alcohol dependence, or an IQ below 80 were excluded. The patients were recruited from various outpatient and inpatient clinics; treatment setting was unrelated to age. The correlation of outcome (defined by the logarithmic transformed ratio of the cumulative months of hospitalization and the cumulative months of illness since the appearance of symptoms) with age was not significant (r=0.01, p=0.88).
The presence or absence of psychopathology was established for all subjects by using the Comprehensive Assessment of Symptoms and History
(26) and the Schedule for Affective Disorders and Schizophrenia
(27) and was assessed by two independent raters. Diagnostic consensus was achieved in the presence of a psychiatrist (e.g., R.S.K.). All of the patients met the DSM-IV criteria for schizophrenia or schizophreniform disorder; those with schizophreniform disorder met the criteria for a diagnosis of schizophrenia after 1 year of illness. All of the healthy comparison subjects met the Research Diagnostic Criteria
(28) for “never mentally ill” and had no first-degree family member with a mental illness. “Age at onset of illness” was defined as the first time the patients had sought medical or psychological help for their psychotic symptoms. All of the patients had received antipsychotic medication in the past, and all but four of the patients had received antipsychotics at the time of the magnetic resonance imaging (MRI) scan. Patient medication included typical and atypical (clozapine, risperidone, olanzapine, and sertindole) antipsychotics. The comparison subjects were matched with the patients for the socioeconomic status of their parents, which was expressed as the highest level of education completed by one of the parents. (See
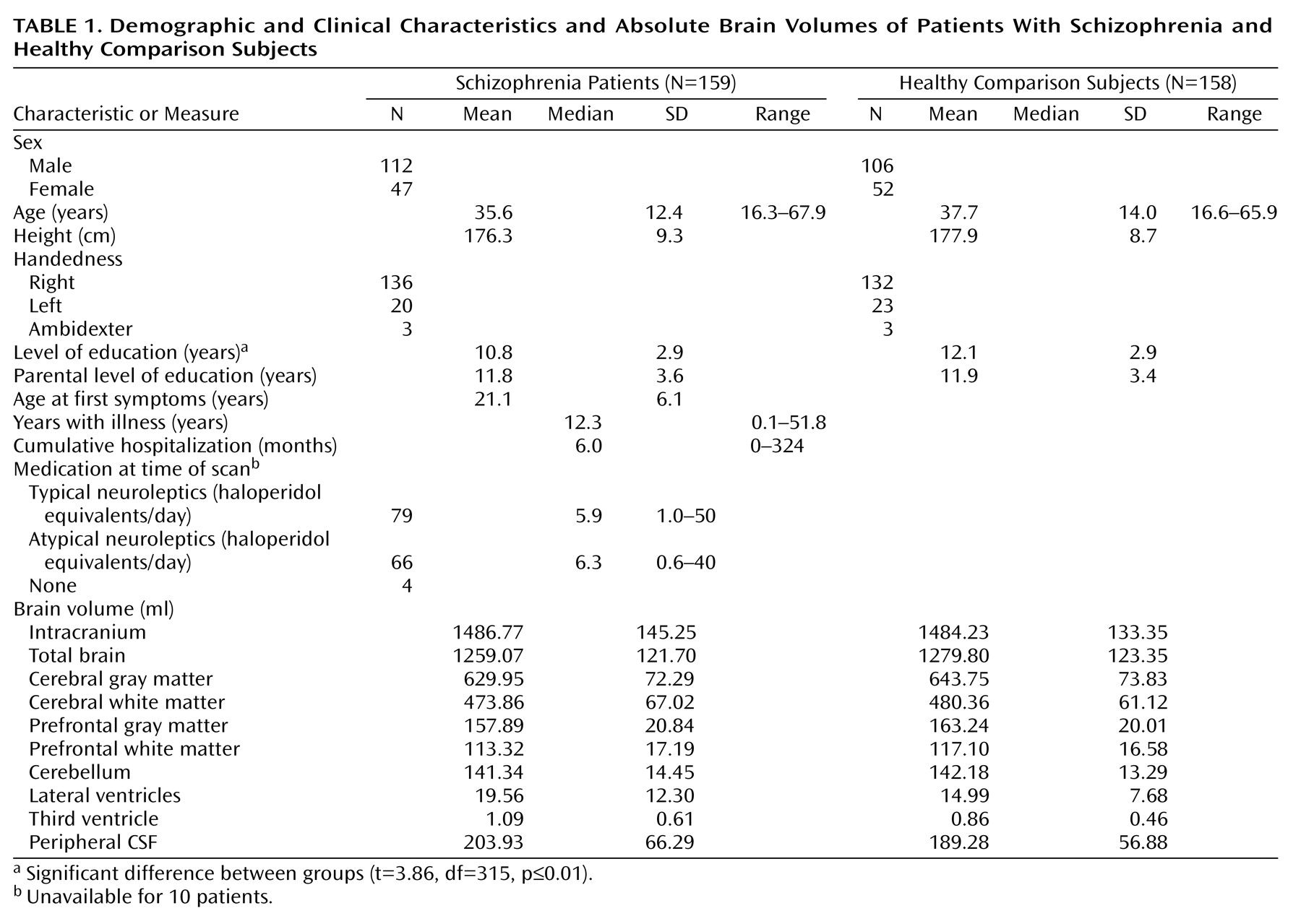
Table 1 for demographic characteristics.) The MRI scans from all subjects were evaluated by two independent clinical neuroradiologists. No gross abnormalities were reported in any of the subjects.
Brain Imaging
MRIs were acquired on a Philips NT (Best, the Netherlands) scanner operating at 1.5 T for all subjects. A three-dimensional fast field echo (TE=4.6 msec, TR=30 msec, flip angle=30°, field of view=256×256 mm2) scan with 160–180 contiguous coronal 1.2-mm slices and a T2-weighted dual echo–turbo spin echo (TE1=14 msec, TE2=80 msec, TR=6350 msec, flip angle=90°, field of view=256×256 mm2) scan with 120 contiguous coronal 1.6-mm slices of the whole head were used for the quantitative measurements. In addition, a T2-weighted dual echo–turbo spin echo (TE1=9 msec, TE2=100 msec, flip angle=90°, field of view=250×250 mm2) scan with 17 axial 5-mm slices and 1.2-mm gap of the whole head was acquired for clinical neurodiagnostic evaluation. Processing was done on the neuroimaging computer network of the Department of Psychiatry at the University Medical Center Utrecht. All images were coded to ensure investigator blindness to subject identification and diagnosis; scans were put into Talairach frames without scaling and corrected for inhomogeneities in the magnetic field.
Quantitative assessments of intracranial, total brain, gray and white matter of the cerebrum (total brain excluding the cerebellum and stem), lateral and third ventricles, and peripheral CSF volumes were performed on the basis of histogram analyses and series of mathematical morphological operators to connect all voxels of interest; they were validated previously
(29). A plane intersecting the fourth ventricle and the aqueduct delimited the cerebellum. In lateral ventricle segmentation, automatic decision rules bridged connections that were not detectable and prevented “leaking” into the cisterns
(30). Coronal slices clearly showing the anterior and posterior commissures delimited the third ventricle; the upper boundary was a plane intersecting the plexus choroideus ventriculi tertii that was perpendicular to the midsagittal slice. All images were checked after measurement and corrected manually if necessary by using Analyze
(31). Segmentation of the frontal lobes was performed automatically by using the ANIMAL anatomical segmentation algorithm
(32), which has been validated previously for volume measurement of the frontal lobe
(33). The interrater reliability of the volume measurements determined by the intraclass correlation coefficient in 10 brains was at least 0.95. (See
Table 1 for mean brain volumes.)
Statistical Analysis
Data were examined for outliers, extreme values, and the normality of the distribution. There was no need for transformation, except for lateral and third ventricle volumes, peripheral CSF volume, and outcome. These variables were normalized by using logarithmic transformations. Multiple linear least-squares regressions of total brain, gray and white matter of the cerebrum, frontal lobe, cerebellum, and lateral and third ventricles, and peripheral CSF volumes were performed. Group (schizophrenia patients or comparison subjects) and sex (men or women) entered the analysis as predictor variables, and intracranial volume and age were entered as covariates. Results from regression equations were expressed as unstandardized (raw) regression coefficients (b). Results from regression equations based on the logarithmically transformed variables could no longer be expressed as mean differences between groups. Instead, they were expressed as the ratio between groups as described in terms of their multiplication factor and 95% confidence interval (CI). The multiplication factor was calculated by exponentiation of b and its lower and upper confidence limits, in which b was the regression coefficient of the logarithmically transformed dependent variable for the group.
To evaluate interactions with age, similar multiple linear least-squares regressions were performed by adding the interaction between age and group as a predictor variable. Results were expressed as a regression slope (b), indicating the changes in volume differences between the patients with schizophrenia and the healthy comparison subjects in the older subjects relative to the younger subjects. In case of a significant main or interaction effect for group, post hoc analyses were performed
1. For side (left or right) by adding handedness (right, left, or ambidexter) as a predictor variable.
2. By adding antipsychotic medication dose or outcome as a predictor variable to the regression analysis for the patients.
3. (For interaction effects with age only) by adding the standard deviation of the unstandardized residual of the dependent variable per age decade as a weight in weighted linear regression analysis to exclude the cohort-biasing effects of the cross-sectional design.
4. By adding time of the scan to the analysis to exclude artifacts due to time of measurement.
The SPSS 9.0 statistical package for Windows (SPSS, Inc., Chicago) was used for these analyses, with a two-tailed alpha level of 0.05.
Results
Table 1 presents the mean brain volumes for the patients with schizophrenia and the healthy comparison subjects.
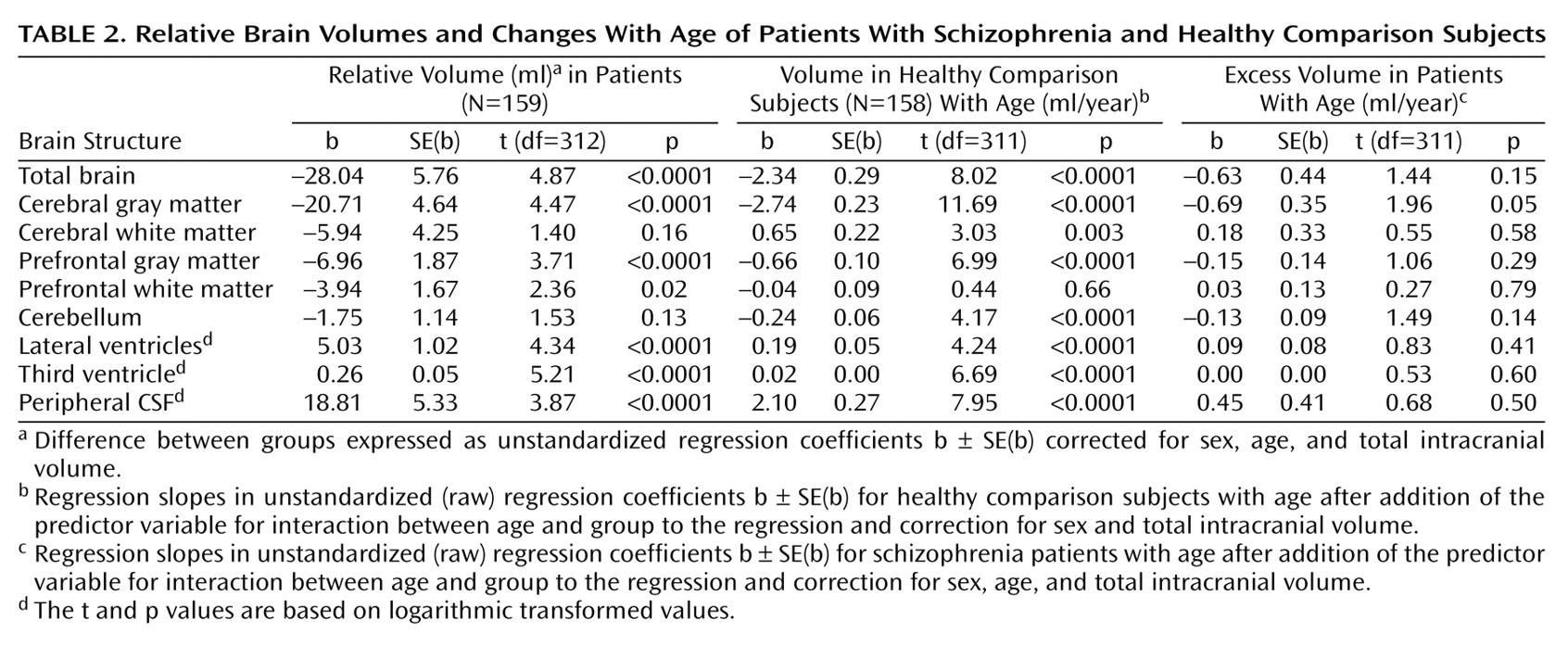
Table 2 and
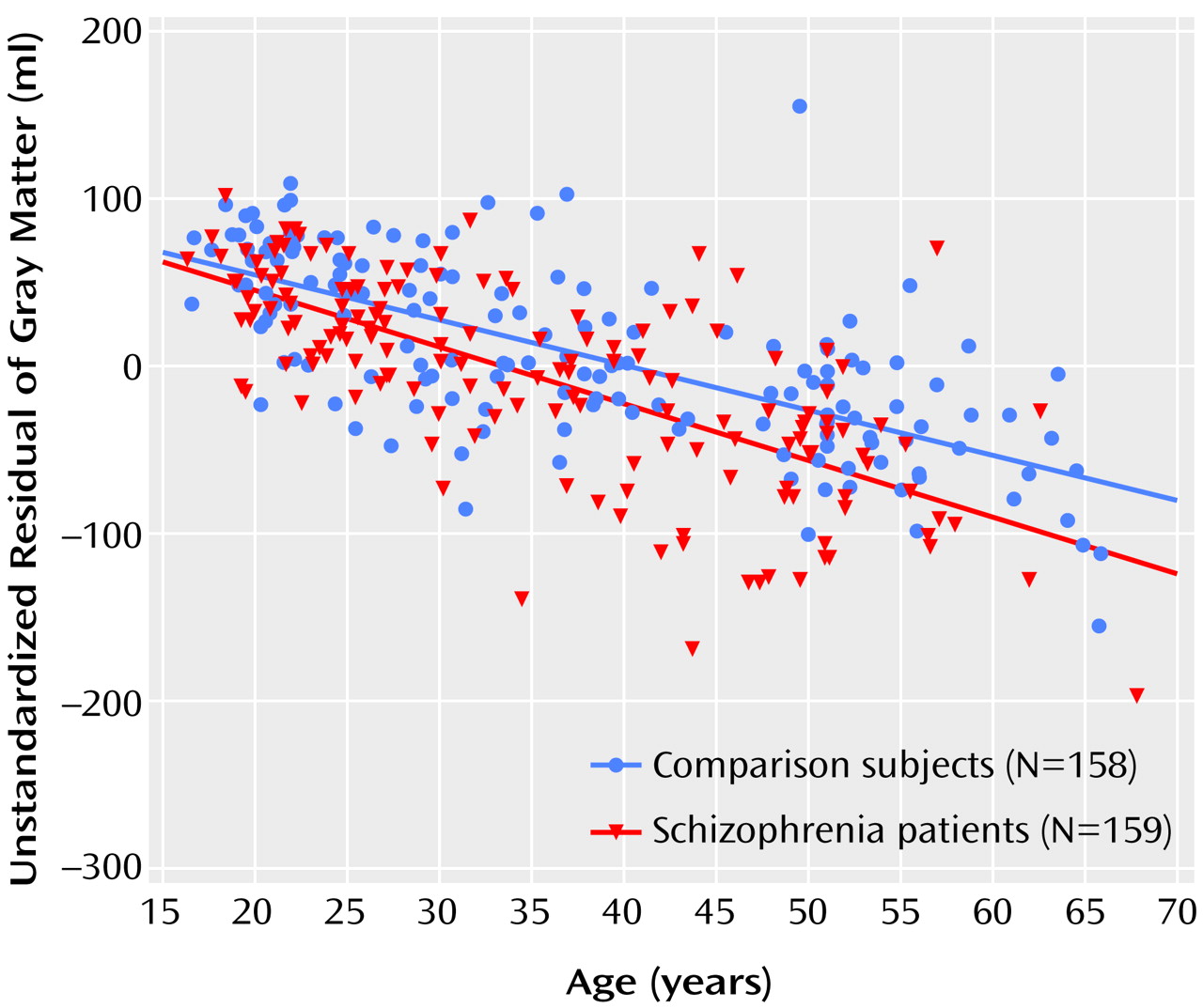
Figure 1 present the main results from the linear regression procedures.
Effects of Diagnosis
Total brain (regression coefficient [b]=–28.04 ml, SE=5.76; t=4.87, df=312, p<0.0001, representing –2.2% smaller total brain volume after correction for sex, age, and intracranial volume), cerebral gray matter (b=–20.71 ml, SD=4.64; t=4.47, df=312, p<0.0001; –3.3% smaller), prefrontal gray matter (b=–6.96 ml, SE=1.87; t=3.71, df=312, p<0.0001; –4.4% smaller), and prefrontal white matter (b=–3.94 ml, SE=1.67; t=2.36, df=312, p=0.02; –3.5% smaller) volumes were significantly smaller in the schizophrenia patients than in the comparison subjects. Lateral ventricle (multiplication factor exponent b=1.27, 95% CI=1.21–1.35; t=4.34, df=312, p<0.0001, representing 27% larger volume), third ventricle (exponent b=1.30, 95% CI=1.24–1.37; t=5.21, df=312, p<0.0001; 30% larger), and peripheral CSF (exponent b=1.11, 95% CI=1.08–1.14; t=3.87, df=312, p<0.0001; 11% larger) volumes were significantly larger in the schizophrenic patients than in the healthy comparison subjects. White matter and cerebellar volumes did not differ between the patients and the healthy comparison subjects. There were no effects of side on any of these findings. Medication at the time of the scan did not explain the volume changes in the patients.
Main Effects of Age
For the healthy comparison subjects, volumes of the total brain, cerebral and prefrontal gray matter, and cerebellum were significantly smaller in the older subjects. Volumes of the white matter, lateral and third ventricles, and peripheral CSF were significantly larger in the older subjects.
Effects of Diagnosis and Age
There was a significant group-by-age interaction for gray matter volume due to a steeper regression slope between age and gray matter volume in the patients with schizophrenia (regression slope [b]=–0.69 ml/year, SE=0.35; t=1.96, df=311, p=0.05, representing a difference in increase in volume of 0.69 ml/year and thus a total volume decrease of –3.43 ml/year) than in the healthy comparison subjects (b=–2.74 ml/year, SE=0.23; t=11.69, df=311, p<0.0001) (
Figure 1). The few outliers in
Figure 1 did not influence the results.
There were no effects for side on gray matter volume. No other significant interaction effects for schizophrenia and age were found. Outcome and antipsychotic medication at the time of the scan did not explain the regression of gray matter volume with age. The standard deviation of the unstandardized residual of gray matter volume per age decade and time of the scan did not influence the interaction effect.
Discussion
This cross-sectional study compared the brain morphology of 159 patients with schizophrenia and 158 healthy comparison subjects across a 55-year age range. The main finding was the more pronounced decrease in gray matter volume in the older patients with schizophrenia. Moreover, irrespective of age, volumes of the total brain and of the cerebral and prefrontal gray matter were smaller, while volumes of the lateral and third ventricles and peripheral CSF were larger in the schizophrenic patients than in the healthy comparison subjects. The effects could not be explained by outcome, antipsychotic medication at the time of the scan, variations of gray matter volume with age, or time-of-measurement effects, except for the greater lateral and third ventricle volumes in the schizophrenic patients, which were associated with poor outcome.
Our results are consistent with those of longitudinal studies that reported progressive decreases in frontal and superior temporal gray matter volumes in patients with chronic schizophrenia
(12), as well as in adolescents with childhood-onset schizophrenia
(13). Our findings also appear to be in agreement with the cognitive and functional decline reported in schizophrenia patients who were chronically ill
(4). Moreover, the findings are consistent with an increase in the severity of negative symptoms
(3) and a decrease in abstract thinking
(5) in older, as compared to younger, patients with schizophrenia.
Irrespective of age, cerebral and prefrontal gray matter volumes were smaller in the patients with schizophrenia. This finding replicates those of earlier studies reporting smaller volumes of cerebral gray matter in neuroleptic-naive patients
(34–
36) and in patients with chronic schizophrenia
(6,
37–
41) that were unrelated to age at onset
(42). The smaller volumes of the total brain and frontal lobe and the larger volumes of the lateral and third ventricles and peripheral CSF are consistent with the majority of the results from earlier reports. Indeed, the extent of the volume differences was quite compatible with the differences reported in a recent meta-analysis of volumetric MRI studies in patients with schizophrenia
(7).
The effects of age on total brain, gray matter, cerebellar, and lateral and third ventricular volumes found in the healthy subjects in this study are consistent with previous findings
(43–
48). They imply that with advancing age, brain volumes decrease and CSF volumes increase. The increased white matter volume found in the older subjects in this study—although small at 0.65 ml/year—was somewhat unexpected because previously no changes
(43,
44,
47) or decreases in volume with age
(49) were reported in adults. However, recently, white matter volume increases up until the fourth decade
(50) have been reported. Since we included subjects who were 16 years and older, our finding was probably due to the inclusion of younger subjects. Indeed, when two separate lines were fitted into the regression analysis
(51), the regression slope for white matter volume and age was steeper in the younger (age ≤25 years) than in the older subjects, although neither reached statistical significance.
One may speculate as to the nature of the more pronounced decrease in gray matter volume in the older patients with schizophrenia. A degenerative process is possible; one could hypothesize that the reported decreases in neuropil
(52,
53), synaptophysin immunoreactivity
(54), and dendritic spine density
(55), as well as cell loss
(56,
57), in patients with schizophrenia are progressive during the course of the illness. Progressive synaptic degeneration has been reported in the left thalamus in schizophrenia patients in a postmortem study
(58). Moreover, it could be argued that processes that are related to normal aging of the brain may go awry in schizophrenia patients. Other, neurochemical, abnormalities that have been proposed in schizophrenia patients may also be implicated
(24). Obviously, it cannot be ignored that an unhealthy lifestyle in schizophrenia patients contributed to our findings; higher rates of smoking and nicotine addiction
(59) as well as poor nutrition
(60) have been reported.
This study was limited in several respects; those should be taken into consideration when interpreting its findings. First, its design was not longitudinal. Therefore, the age-related findings were potentially confounded by cohort or time-of-measurement effects
(61). Although effects from cohort or time of measurement cannot be excluded completely, we analyzed some of the possible confounders to assess these effects as much as possible. Outcome and age at onset of the disease did not influence the gray matter changes with age. Changes in gray matter variance with age and time of the scan did not affect the results. All volume measures were corrected for intracranial volume, which remains stable with age. Second, medication may have influenced our findings in patients
(62). Although antipsychotic medication taken at the time of the scan was not related to the excessive decrease in gray matter volume with age, the effects of cumulative years of medication treatment cannot be ruled out. However, no clear effects of antipsychotics on brain morphology have been found in earlier studies
(24).
This study confirmed that the smaller brains of patients with schizophrenia could be explained by smaller gray matter volumes. Moreover, the more pronounced decreases in gray matter volume in the older patients with schizophrenia may suggest a progressive loss of cerebral gray matter with schizophrenia. However, the effects of cumulative antipsychotic medication and an unhealthy lifestyle due to higher rates of smoking and nicotine addition as well as poor nutrition in the schizophrenia patients must be considered as alternative explanations.