Epidemiological studies have suggested that mild depressive syndromes are common among elderly men
(1). Dysthymic disorder is the best-described mild chronic depressive syndrome. Another condition that is common among elderly men is hypogonadism (or testosterone deficiency)
(2), and many psychiatric symptoms of hypogonadism (e.g., fatigue, loss of libido, irritability, and dysphoria) overlap with dysthymic disorder. To our knowledge, the relationship between hypogonadism and dysthymic disorder has not been systematically investigated in elderly men.
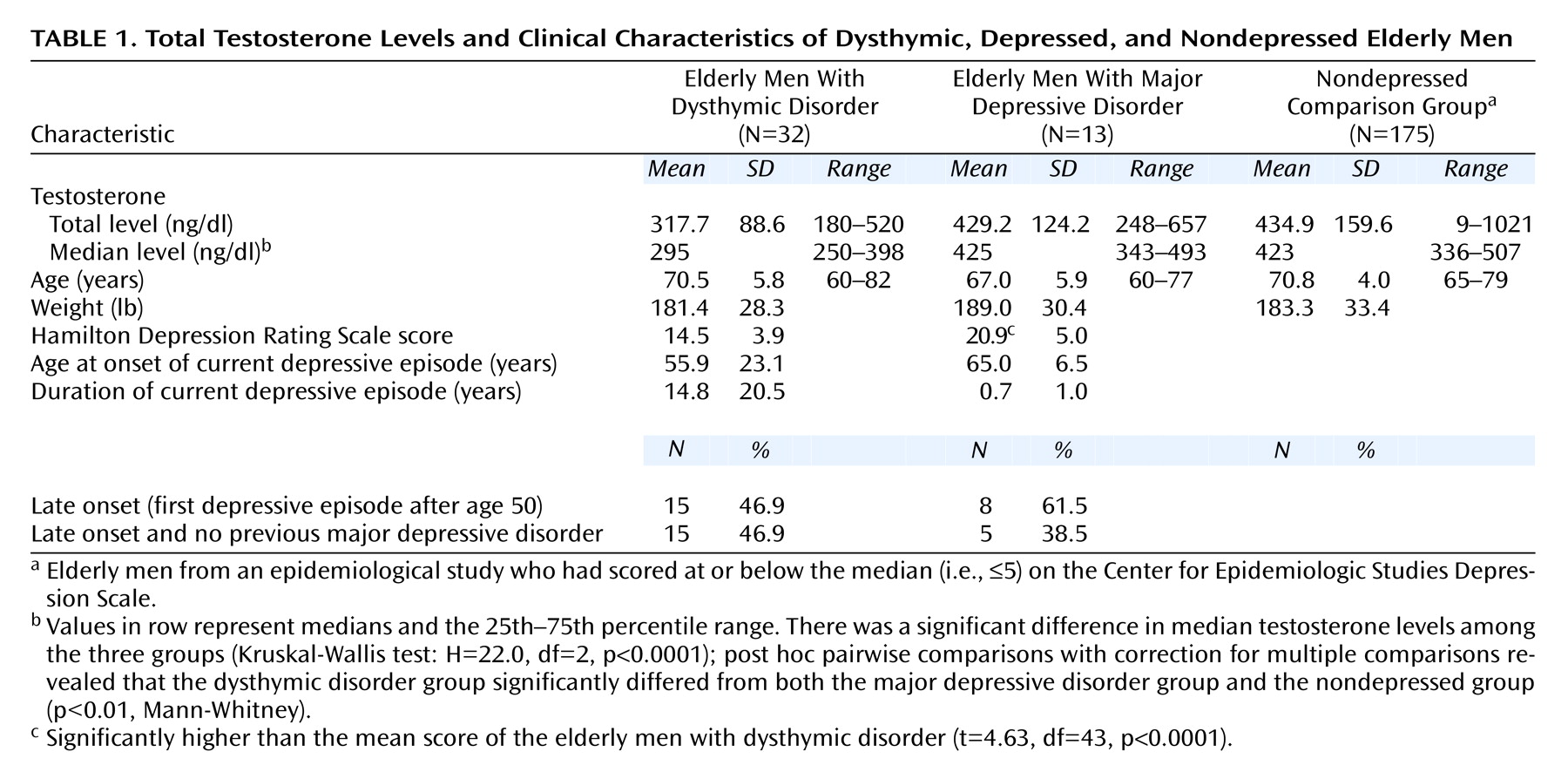
We hypothesized that lower testosterone levels may be etiologically important in the development of this depressive syndrome in elderly men. As a preliminary test of the hypothesis that older men with dysthymic disorder have hypothalamic-pituitary-gonadal (HPG) axis hypofunctioning, we measured total testosterone levels in a clinical patient group of elderly men with dysthymic disorder and compared them to testosterone levels from the following two groups: 1) age-matched men with major depressive disorder who enrolled in a clinical trial at the same geriatric depression clinic and 2) age-matched nondepressed men who participated in a large epidemiological study of male aging.
Method
Depressed patients were evaluated at the Late Life Depression Clinic of the New York State Psychiatric Institute, a specialty clinic dedicated to research in depressed patients ≥60 years old. The majority of patients were recruited by advertisements that offered free evaluation and free treatment for patients eligible for research studies. Patients were thoroughly evaluated by a psychiatrist; a research social worker independently conducted the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)
(3). Any discrepancy between the psychiatrist’s clinical diagnosis and the SCID diagnosis was resolved at a consensus staff conference.
Patients who met DSM-IV criteria for dysthymic disorder were eligible for a 12-week, double-blind clinical trial in which they received either fluoxetine or placebo. Patients who met DSM-IV criteria for major depressive disorder and who had no ischemic heart disease were eligible for a 12-week, double-blind clinical trial in which they received either sertraline or nortriptyline. All patients signed informed consent for study participation. In both studies, blood was drawn between 9:00 a.m. and 3:00 p.m. at week 2–3 of the study for the primary purpose of determining medication level. The sample was stored for testosterone assay at –70°C. Plasma testosterone level was determined by the stable isotope dilution technique, with deuterated testosterone used as the internal standard.
Our nondepressed subjects came from the Massachusetts Male Aging Study, a prospective observational study of health in randomly selected men
(4). A total of 1,709 of 3,258 eligible respondents completed the baseline in-home protocol. The follow-up phase of the Massachusetts Male Aging Study was conducted from 1995 to 1997. Of the 1,496 respondents eligible for follow-up, 1,156 completed a follow-up interview (77% follow-up response rate).
A trained field technician/phlebotomist visited each subject in his home. Each subject completed the Center for Epidemiologic Studies Depression Scale (CES-D)
(5), a 20-item self-report inventory that measures current level of depressive symptoms in community populations. Scores can range from 0 to 60, with higher scores indicating more depressive symptoms. Two blood samples were drawn 30 minutes apart within 4 hours of the subject’s awakening. Blood was kept in an ice-cooled container for transport and centrifuged within 6 hours. Serum was shipped to the laboratory at the University of Massachusetts in Worcester within 1 week by same-day courier and stored at –70°C until time of assay. The tubes of blood were pooled for analysis in equal aliquots to smooth episodic secretion, and total testosterone was measured by using a radioimmunoassay kit (Diagnostic Products Corp., Los Angeles).
From this study a subgroup of 175 nondepressed elderly men was selected as a comparison group by using the following criteria. First, men older than 65 were selected (mean age=70.8 years, range=65–79 years; N=499). The median CES-D score of this group was 5. Using an a priori definition of “nondepressed” as those with CES-D scores at or below the median, we selected the men with scores ≤5 (N=258) and further restricted the group to include only those men with complete information on age, weight, CES-D score, and total testosterone level (N=175).
Frequencies and means were used to describe discrete and continuous data, respectively. A Kruskal-Wallis test, which is a nonparametric location test, was used to test for differences in central tendency among the three groups. Post hoc (Mann-Whitney) pairwise comparisons with correction for multiple comparisons were used to make inferences about how median testosterone levels differed between groups (overall type I error rate for all three comparisons, alpha=0.05).
Discussion
To our knowledge, this is the first study that assessed the relation between testosterone level and mild or chronic depressive illness in elderly men. We found that elderly men with dysthymic disorder had significantly lower total testosterone levels than did men with major depressive disorder and men without depression. Furthermore, we found that a majority of the elderly men with dysthymic disorder had total testosterone levels in the hypogonadal range (i.e., ≤300 ng/dl).
Several methodological limitations to the current study should be noted. One concerns the delineation of the comparison group, i.e., the criterion we used to define “nondepression” (below-median CES-D score). Another possible confound is the use of a cross-laboratory comparison, which is especially problematic in the absence of between-laboratory validation studies. Of note in this regard is that the testosterone levels of the subjects from the Massachusetts Male Aging Study and the elderly men with major depressive disorder from the present study are consistent with data from a comprehensive meta-analysis of 44 well-designed studies
(2) that reported a mean testosterone level in adult men of 479 ng/dl. A final limitation concerns the problems inherent in using one total testosterone measurement to reflect HPG axis functioning, since 1) androgenic effects may be best predicted by the unbound or free testosterone fraction
(6), and 2) testosterone levels are influenced by multiple factors (e.g., diurnal variation, obesity, the experience of defeat, diet)
(7,
8). We chose to use a single total testosterone measurement because it is a reliable measure of testosterone status in this population. In a study that included eight testosterone levels over 1 year in 169 middle-aged and elderly men, the first sample was highly correlated (r=0.90) with the annual mean testosterone level of all samples
(9). We did not use free testosterone because this measure may be especially sensitive to the storage time of the serum sample
(10), which could not be controlled for across the study groups. Finally, since diurnal variation is minimal among older men
(7), the lack of a standard time of sampling is unlikely to have affected the results.
The psychiatric symptoms of hypogonadism overlap with symptoms of depression and include low libido, fatigue, loss of confidence, and irritability
(11). Initial interest in this relationship focused on major depressive disorder, i.e., whether men with major depressive disorder had HPG axis abnormalities. The focus then became whether hypogonadal men developed a distinct “secondary” depression that might be reversible with testosterone replacement
(8). However, epidemiological and neuroendocrine studies have suggested that HPG axis dysfunction is not central to major depressive disorder
(8). Furthermore, although anecdotal reports and uncontrolled data suggest that in some hypogonadal men, comorbid major depressive disorder remits with testosterone replacement
(12) or testosterone augmentation to treatment with selective serotonin reuptake inhibitors
(13), data do not support the assumption that testosterone replacement in men with major depressive disorder conforms to the “hypothyroid” model, i.e., hormonal axis normalization as an effective antidepressant. Indeed, we recently completed a double-blind, randomized clinical trial of testosterone replacement versus placebo in 30 men with major depressive disorder and hypogonadism and found that testosterone replacement was indistinguishable from placebo in antidepressant efficacy
(14).
The results of the current study suggest the possibility that HPG axis hypofunctioning is related to a chronic, low-grade depressive syndrome in some elderly men. Such an association could be the result of either chronic depression leading to HPG axis blunting or to HPG axis hypofunctioning leading to low-grade depression. The possibility that a long duration of depressive symptoms leads to hypogonadism has not been studied, although the transient experience of defeat and submission has a well-established negative impact on testosterone level
(8). Notably, the dysthymic men in our study had a mean duration of depressive illness of almost 15 years, compared with less than 1 year for the men with major depressive disorder. One explanation for our findings, therefore, could be that the chronicity of depressive illness leads to low testosterone level. In this context, it would be informative to study testosterone levels in men with chronic major depressive disorder.
An alternative explanation for our results is that the normative, age-related decline in testosterone level—perhaps below a threshold testosterone level, in terms of relative change from baseline, or in vulnerable subpopulations—can lead to mild, persistent depressive symptoms. If HPG axis blunting does lead to dysthymic disorder in vulnerable individuals, then the population-wide, age-related testosterone level decline would argue for an increasing prevalence of new-onset dysthymic disorder in middle-aged and elderly men. Accumulating clinical and epidemiological data support this possibility. For example, in a well-described group of 40 elderly patients with dysthymia, Devanand and colleagues
(15) reported that the sex ratio was 1:1 and that few patients had a comorbid personality disorder (10%) or a prior episode of major depressive disorder (20%)—in marked contrast to younger patients with dysthymia. Moreover, mean age at onset of dysthymic disorder was 55.2 years (SD=15.4), and the majority had developed dysthymic disorder as their first and only depressive illness in mid-life. This phenomenologic pattern of “late-onset dysthymic disorder” is supported by data from a second group of over 160 elderly dysthymic patients from the same clinic (unpublished 2001 study of D.P. Devanand) and from data collected in a large clinical trial
(16).
The relationship between a mild depressive syndrome and mild HPG axis hypofunctioning, both of which are generally undiagnosed in elderly men, could be easily overlooked. Future studies should focus more specifically on HPG axis functioning in elderly dysthymic men and on the therapeutic role of testosterone replacement.