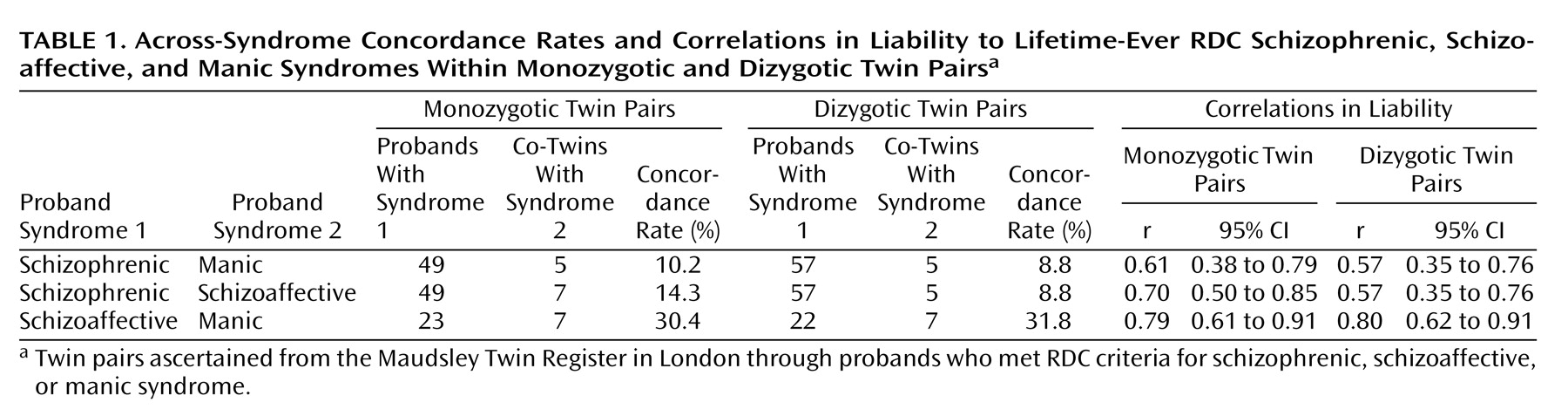
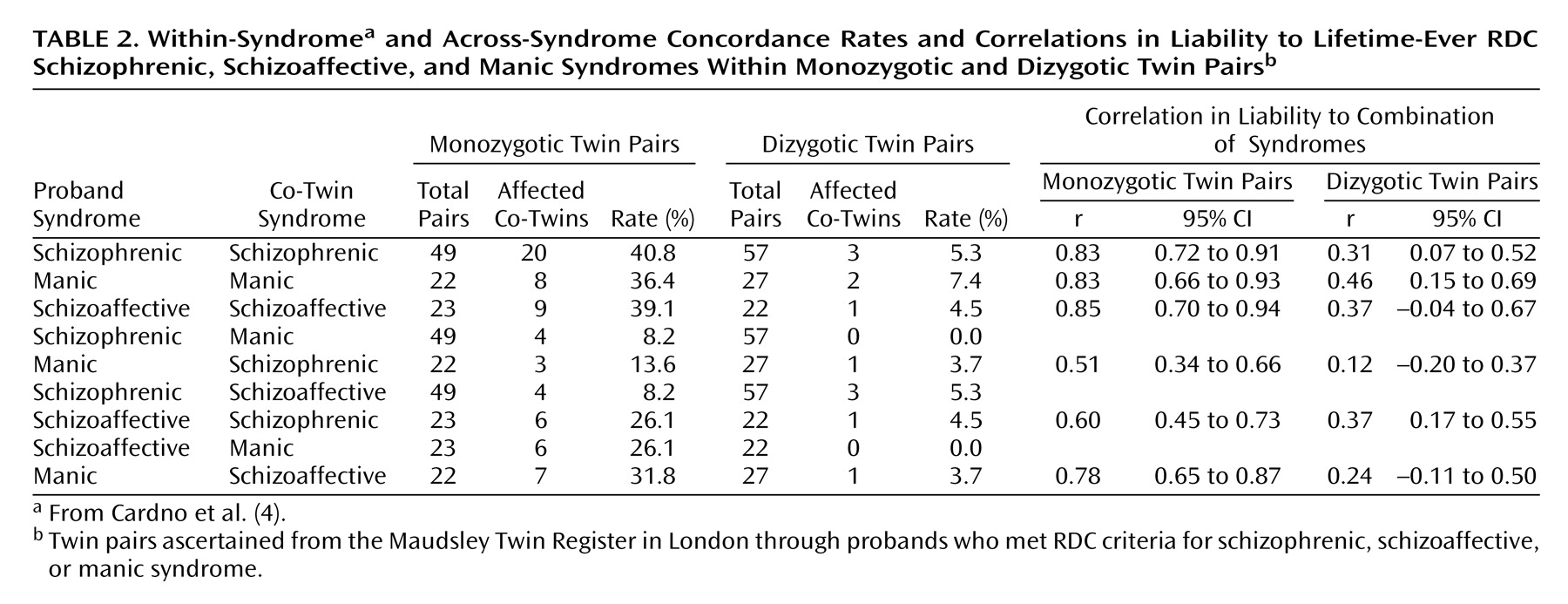
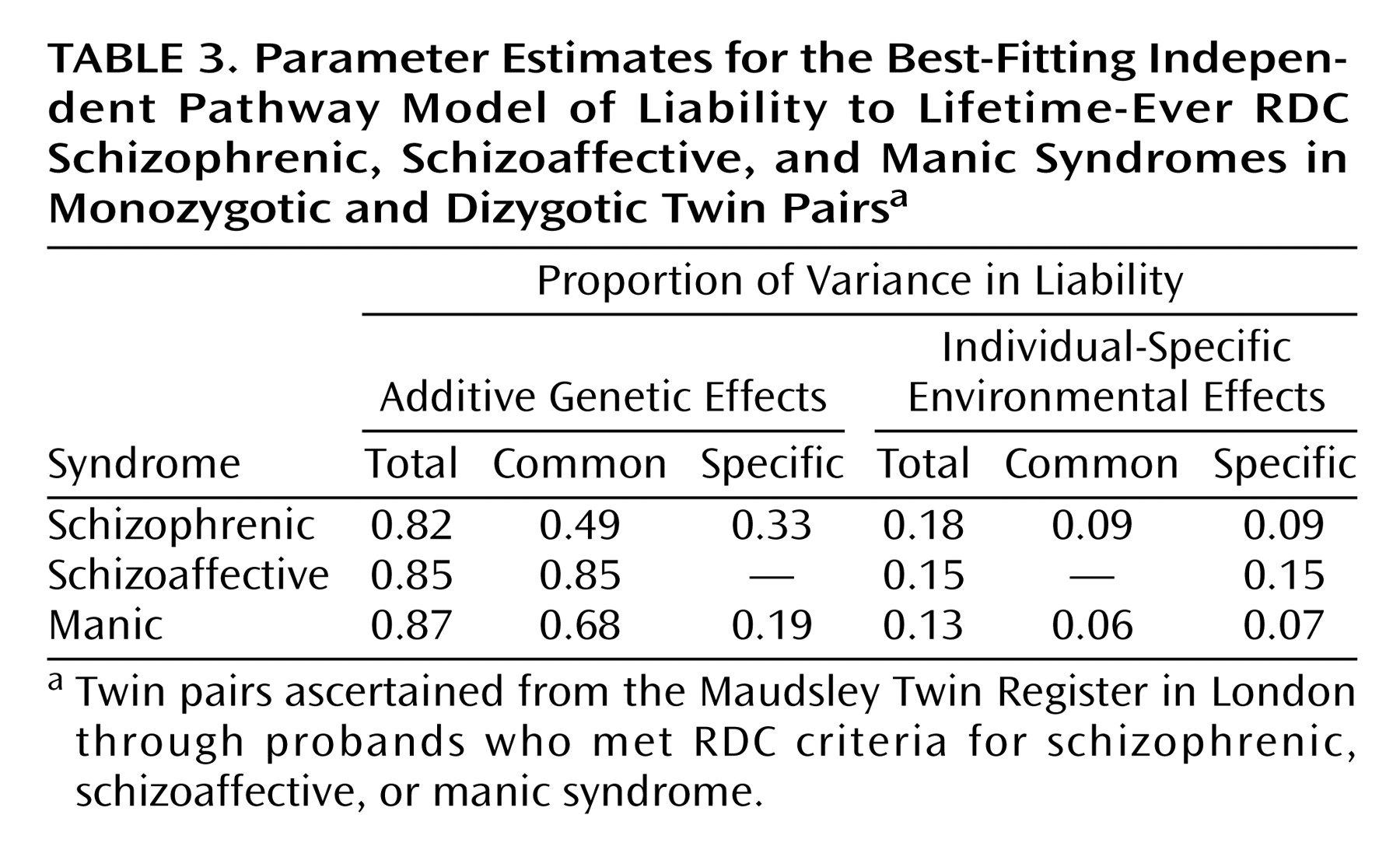
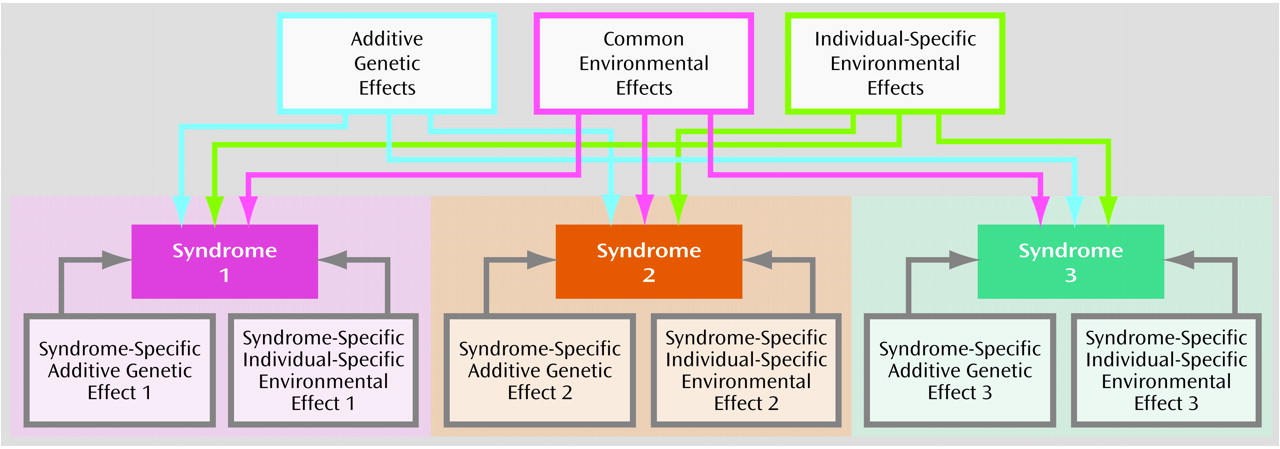
There is evidence for a substantial genetic contribution to the etiology of schizophrenia, schizoaffective disorder, and mania/bipolar disorder, with heritability estimates for operational diagnoses ranging from 70% to 85%
(1–
4). An understanding of the extent to which these disorders share genetic and environmental risk factors is important for the classification of the psychoses as well as for attempts to locate and identify genes that contribute to psychoses.
There is evidence of familial co-aggregation between schizoaffective disorder and both schizophrenia and bipolar disorder; however, no familial relationship has generally been found between schizophrenia and bipolar disorder
(5–
8). In addition, it is unclear from family studies how the relationship of schizoaffective disorder to other psychoses is best conceptualized
(9,
10), and such studies cannot distinguish between genetic and common environmental effects.
An earlier study involving the first half of the Maudsley twin series investigated the occurrence of affective and schizoaffective disorders in co-twins of schizophrenic probands
(2). More recently, we reported heritability estimates in the updated series, looking at twins concordant for particular categories of psychosis
(4). However, studies of individuals of multiple births focusing on concordance for psychosis but discordance for type of psychosis have been confined to reports of single sets of twins or triplets
(11,
12).
Most previous quantitative genetic studies of functional psychoses have employed a phenotypic definition based on a single main lifetime diagnosis. This approach requires a diagnostic hierarchy, with schizophrenia at the top, followed by schizoaffective disorder, then mania/bipolar disorder. If, for example, in the early stages of an illness, an individual shows symptoms that fulfill criteria for a manic episode, but later the clinical presentation becomes typical of schizophrenia, the main lifetime diagnosis is regarded as schizophrenia, and the earlier manic symptoms are regarded as nonspecific and not considered in further analyses.
An alternative hypothesis is that the occurrence of manic episodes in individuals with a main lifetime diagnosis of schizophrenia indicates that the two clinical syndromes share some risk factors in common. This hypothesis predicts that, when psychotic syndromes are defined nonhierarchically (i.e., allowing individuals to fulfill criteria for more than one syndrome in their lifetime): 1) schizophrenic and manic syndromes will co-occur in individuals more frequently than expected according to the population prevalences of these syndromes; 2) if there is an overlap in familial risk factors, then in a group of twin pairs, co-twins of probands with schizophrenic syndromes will have manic syndromes more frequently than expected (and vice versa); and 3) if there is an overlap in genetic risk factors, this comorbidity within twin pairs will be greater for monozygotic than dizygotic pairs
(13).
In contrast, if the occurrence of manic episodes in individuals with a main lifetime diagnosis of schizophrenia is nonspecific, there may be significant within-person comorbidity (prediction 1), but there should be no effect on familial comorbidity (predictions 2 and 3) and hence no evidence of an overlap in environmental or genetic risk factors between the syndromes. These hypotheses can be tested by a process of biometrical model fitting, which formally investigates whether, and to what extent, genetic and environmental effects are shared between phenotypes
(14).
Relaxing the constraint imposed by a diagnostic hierarchy is a relatively novel way of investigating genetic influences on psychoses; however, it has proved useful in the study of other psychiatric disorders, for example, by showing an overlap in genetic risk factors for major depression and generalized anxiety disorder
(13).
The objective of this study was to investigate whether the genetic and environmental liabilities to operationally defined schizophrenic, schizoaffective, and manic syndromes overlap in a group of twins that was systematically ascertained through probands with any functional psychosis. We use the term psychotic “syndrome” to refer to the cross-sectional occurrence of a group of symptoms, as distinct from the concept of a psychotic “disorder,” which, in genetic studies, generally refers to a single diagnosis based on the predominant clinical features shown by an individual during his or her lifetime.
Method
Subjects
The study group has been described in detail elsewhere
(4). Briefly, probands were ascertained from the Maudsley Twin Register in London, as patients of multiple birth who had attended any facility of the Maudsley and Bethlem Royal Hospitals between 1948 and 1993, who had a same-sex co-twin surviving to 15 years of age, and who had psychotic symptoms (following the inclusion criteria of Sartorius and colleagues
[15]) or an episode of Research Diagnostic Criteria (RDC) mania or hypomania
(16) at some time in their lives. Probands were excluded if they had a primary diagnosis of dementia or if psychotic symptoms occurred only during acute organic states.
Zygosity determination was carried out blind to research diagnoses and was based on all available information, including analysis of genetic markers in 42.4% of pairs. Agreement between zygosity determination by genetic markers and by resemblance information was 95.2%.
The study group comprised 224 probandwise pairs (120 male and 104 female pairs), from which 28 probands (12.5%) were doubly ascertained after checking for independence of ascertainment. A total of 197 pairs (87.9%) were white. The mean age of the co-twins at last follow-up was 46.5 years (SD=15.4, range=15–88), and 64 co-twins (28.6%) were followed up beyond 55 years of age. The equal environments assumption was supported in this study group by an absence of positive correlations between degree of physical resemblance or length of cohabitation of monozygotic twins and concordance for psychosis. There was no substantial ascertainment bias for zygosity (106 monozygotic and 118 same-sex dizygotic pairs), but the study group may have contained subjects with more severe disorders than if we had ascertained through a population-based sample
(4).
Clinical Assessment
Clinical assessment was based on all available clinical information, including a research interview (with Gottesman and Shields’s cued-questions interview
[17] and/or the Schedule for Affective Disorders and Schizophrenia—Lifetime Version
[18]) in 72.8% of probands and 59.4% of co-twins. Interviewed twins gave verbal consent to be studied. Written informed consent was not required by the local ethics committee at the time the study was instituted.
The assessment of RDC lifetime-ever psychotic syndromes was made by separate raters for each member of concordant twin pairs. A nonhierarchical approach was used, and individual twins could qualify for more than one psychotic syndrome on a lifetime basis. Interrater reliability was assessed by using data for 30 subjects and expressed as the mean kappa coefficient between raters. Kappa was 0.64 for RDC schizophrenic syndrome, 0.58 for schizoaffective syndrome, and 0.68 for manic syndrome. The quality of clinical information was higher for co-twins of probands with a schizophrenic and schizoaffective syndrome than with a manic syndrome
(4), which could cause a relative underestimate of heritability for the manic syndrome. Details of probandwise concordance rates and heritability estimates for the syndromes have been reported elsewhere
(4).
Statistical Analysis
Concordance rates and correlations in liability
Comorbidity was initially investigated for the three pairings of schizophrenic syndrome with manic syndrome, schizophrenic syndrome with schizoaffective syndrome, and schizoaffective syndrome with manic syndrome. Probandwise concordance rates in monozygotic and dizygotic twins were calculated across syndromes 1) within twin probands (because twins could qualify for more than one syndrome during their lifetime), and 2) within twin pairs. Correlations in liability were also calculated for each combination using the Mx program
(19). The correlations in liability are tetrachoric correlations that are based on a liability-threshold model
(20) that makes use of concordance rates and population morbidity risks for the syndromes. In the current study, population risks were derived from local case register data for schizophrenia
(4) and were not direct population-based estimates. However, the resulting heritability estimates for psychotic syndromes
(4) were very similar to those from studies that estimated population risks directly
(3,
21). The morbidity risk estimates and 95% confidence intervals (CIs) were 0.82% (95% CI=0.69–1.04) for the schizophrenic syndrome, 0.35% (95% CI=0.25–0.51) for the schizoaffective syndrome, and 0.38% (95% CI=0.27–0.55) for the manic syndrome.
If there is overlap for any kind of risk factor, the correlations in liability within probands should be statistically significant and similar in monozygotic and dizygotic probands. Beyond this, different patterns of correlations would be expected according to the type of risk factors causing the comorbidity
(13), as follows:
1. If individual-specific environmental factors cause the comorbidity, significant within-proband correlations only would be expected.
2. If common environmental factors cause the comorbidity, significant correlations within probands and also within pairs, with similar correlations in monozygotic and dizygotic pairs, would be expected.
3. If additive genetic factors cause the comorbidity, significant correlations within probands and also within pairs, with the correlation twice as great in monozygotic than dizygotic pairs, would be expected.
If comorbidity of syndromes occurs because of a combination of these factors, the pattern of correlations in liability would be expected to reflect the relative prominence of the different effects.
Independent pathway model
Inspection of the pattern of correlations in liability gives an indication of what type of risk factors are contributing to comorbidity between syndromes. The contribution of risk factors can be formally tested by a process of biometrical model fitting. Considering all three syndromes together allowed the fitting of an independent pathway model
(14) (
Figure 1), in which the variance in liability to the three syndromes was partitioned into genetic and environmental components that are shared in common by the syndromes (additive genetic effects, common environmental effects, and individual-specific environmental effects) and residual genetic and environmental components that are specific to each syndrome (syndrome-specific additive genetic effects for each of the three syndromes and syndrome-specific individual-specific environmental effects for each of the three syndromes).
The independent pathway model is based on a liability-threshold model in which the liabilities to the three syndromes are assumed to have a joint distribution that is multivariate normal. The twin data were tabulated in the form of contingency tables for monozygotic and dizygotic pairs. The tables give the frequencies of all possible combinations of illness status (all combinations of either syndrome present or absent in probands and co-twins). In this study, ascertainment was probandwise (i.e., all probands were affected), so there were 56 combinations represented in eight-by-seven tables. Because of the symmetry of combinations across the main diagonal of the tables
(22), the number of combinations was reduced to 35 observed frequencies for analysis.
Expected proportions in each cell (given the specified thresholds) were calculated by six-dimensional numerical integration of the multivariate normal distribution and evaluated against the observed cell proportions to derive a maximum likelihood correlation matrix for both monozygotic and dizygotic twins. This correlation structure was then decomposed into maximum likelihood genetic and environmental effects on the basis of the specified biometrical genetic model
(14) shown in
Figure 1.
When ascertainment is probandwise, pairs in which neither twin is affected are omitted from observation. This introduces a potential bias that can be adjusted for by dividing the likelihood of the data by the proportion of the population remaining after ascertainment (19). We obtained this adjustment by dividing the expected proportion in each sampled cell of the contingency table by the sum of the expected proportions of these cells (
Appendix 1).
To apply the correct ascertainment strategy to our genetic models, we also estimated π, the probability of being ascertained given that an individual is affected. Under double ascertainment (π=1) all cells of the contingency table will have equal probability (p), whereas under single ascertainment (π→0) concordant pairs are twice as likely to be ascertained (2p) than pairs with only one affected twin (p). The estimated value of a scalar (s) for the concordant cells shows whether there is evidence for double (s=0.5), single (s=1), or other intermediate ascertainment probability (0.5<s<1). Since s did not significantly differ from 1 in our study group, we based the model fitting on single ascertainment.
A goodness-of-fit chi-square statistic was calculated as twice the log-likelihood of the observed data under the model subtracted from twice the log-likelihood of the “saturated model” (i.e., a perfectly fitting model in which the likelihood of the observed data themselves is calculated). Models incorporating additive genetic, common environmental, and individual-specific environmental effects (ACE model) and additive genetic and individual-specific environmental effects only (AE model) were fitted. In our previously reported univariate analysis, the AE model fitted best for all three syndromes, but we could not exclude the possibility of some common environmental effects being present
(4). To account for possible inaccuracies in the morbid risk estimates (liability thresholds), the model fitting was also performed by using the upper and lower extremes of the 95% confidence intervals of the morbid risk estimates for each syndrome.
Results
Concordance Rates and Correlations in Liability
The within-proband across-syndrome concordance rates and correlations in liability are shown in
Table 1. The correlations were statistically significant for all three combinations of syndromes, and correlations in monozygotic and dizygotic probands did not significantly differ. This pattern is consistent with all three pairs of syndromes sharing some risk factors in common. It also shows that level of comorbidity is independent of zygosity.
The within-pair concordance rates and correlations in liability are shown in
Table 2 (the concordance rates and correlations in liability from the univariate analysis
[4] are also given for comparison). The correlations were significant for all combinations of syndromes in monozygotic pairs and were also significant in dizygotic pairs for the combination of the schizophrenic with the schizoaffective syndrome. The correlations in monozygotic pairs were higher than in dizygotic pairs for all combinations of syndromes, but the higher correlation was significantly different only for the combination of the schizoaffective with the manic syndrome. This finding suggests that the risk factors shared by the three pairs of syndromes may include genetic effects.
Independent Pathway Model
The fit of the ACE model (χ
2=70.46, df=56, p=0.09) did not significantly drop when the C structure (common environmental effects) was fixed to zero (χ
2=72.36, df=59, p=0.11), as the difference in chi-square for three degrees of freedom did not exceed the critical value of 7.81 at the 0.05 level. The AE model, therefore, was the best-fitting model on grounds of parsimony. Further inspection of the path estimates of the AE model showed nonsignificant syndrome-specific additive genetic effects and common individual-specific environmental effects for the schizoaffective syndrome (χ
2=75.02, df=61, p=0.11). This result implies that the genetic liability to the schizoaffective syndrome is entirely shared in common with the liability to the other two syndromes but that individual-specific environmental effects are not shared with the other syndromes. The parameter estimates for the best-fitting independent pathway model are shown in
Table 3.
The additive genetic correlations and 95% CIs for each pairing of syndromes were 0.68 (95% CI=0.67–0.73) for schizophrenic with manic syndrome, 0.77 (95% CI=0.67–0.83) for schizophrenic with schizoaffective syndrome, and 0.88 (95% CI=0.79–0.95) for schizoaffective with manic syndrome. The individual-specific environmental correlation and 95% CI was 0.48 (95% CI=0.05–0.93) for schizophrenic with manic syndrome.
When the analyses were repeated by using the upper and lower extremes of the 95% confidence intervals of the morbid risk estimates, the model-fitting results and genetic and environmental correlations showed no substantive changes.
For comparison, we also fitted a common pathway model. This is a more restricted model in which genetic and environmental effects influence syndromes indirectly through an intermediate unobserved variable (e.g., general liability to psychosis). As with the independent pathway model, the AE common pathway model fitted best (χ2=73.38, df=60, p=0.13). The parameter estimates were generally similar to those based on the independent pathway model, including a nonsignificant syndrome-specific effect for the schizoaffective syndrome (χ2=73.44, df=62, p=0.15). However, the common individual-specific environmental factor for the schizoaffective syndrome could not be dropped (change in χ2=4.54, df=1) and had a parameter estimate of 0.07. This difference between the models can probably be accounted for by the underlying assumptions. In the more restricted common pathway model, the individual-specific environmental factor was a single factor influencing the latent variable of general liability to psychosis. Thus, it was impossible to tease out the significance of the separate effects of the common factors on the three syndromes. The independent pathway model, therefore, gives more insight into the genetic and environmental correlational pattern between syndromes.
Discussion
Relationship Between Schizophrenic and Manic Syndromes
The results of this study suggest that the RDC schizophrenic and manic syndromes, defined nonhierarchically on a lifetime-ever basis, share some common genetic risk factors. The two syndromes showed significant comorbidity within probands, and the within-pair correlations were more than twice as great in the monozygotic than in the dizygotic twin pairs, suggesting a genetic contribution to the comorbidity. Consistent with this interpretation, the model fitting showed significant genetic correlations between the schizophrenic and manic syndromes. This result would also be in keeping with the suggestion, based on a review of genetic linkage studies, that schizophrenia and affective disorder share some susceptibility genes
(23). In addition, the results support an overlap in environmental risk factors for the schizophrenic and manic syndromes.
These results differ notably from those of family studies that have defined schizophrenia and bipolar disorder by using a single hierarchical main lifetime diagnosis and that have generally not found significant familial co-aggregation between these two disorders
(5–
8). The independent pathway model tested in this study requires a diagnostic approach that allows within-subject comorbidity. If a diagnostic hierarchy is adopted in the current sample, with RDC schizophrenia above mania, we find no pairs in which one twin has schizophrenia and the other has bipolar disorder (concordance rates: proband with schizophrenia/co-twin with bipolar I disorder or mania, 0 of 49 for monozygotic twins and 0 of 57 for dizygotic twins; proband with bipolar I disorder or mania/co-twin with schizophrenia, 0 of 12 for monozygotic twins and 0 of 15 for dizygotic twins).
However, on examining the underlying comorbidity, we find five monozygotic probands with a main lifetime diagnosis of schizophrenia who also fulfilled criteria for a manic episode at some time in their lives (
Table 1). Two (40.0%) of their co-twins had an episode of mania (in both cases, the co-twin’s main lifetime diagnosis was schizoaffective disorder). Forty-four monozygotic probands with a main lifetime diagnosis of schizophrenia had not had a manic episode, and two (4.5%) of their co-twins had an episode of mania (in both cases, the co-twin’s main lifetime diagnosis was schizophrenia). This difference was statistically significant (p<0.05, Fisher’s exact test). In contrast, none of the five co-twins of probands with schizophrenia plus a manic episode had schizophrenia, while 20 (45.5%) of the 44 co-twins of probands with schizophrenia only had schizophrenia (p=0.06, Fisher’s exact test). Therefore, taking into account whether or not an individual with a main lifetime diagnosis of schizophrenia has also had an episode of mania provides potentially valuable information concerning the pattern of psychotic symptoms in their relatives.
A further issue relates to the fact that the current study group was probably selected for severity
(4). Of particular relevance, 96% of probands with a manic syndrome were rated as having had psychotic symptoms at some time. Therefore, it is unclear whether the manic syndrome as a whole shares genetic risk factors with the schizophrenic syndrome or whether the relationship primarily applies to the manic syndrome accompanied by psychotic symptoms.
Status of the Schizoaffective Syndrome
The results of the current study, which was based on nonhierarchical definition of syndromes, are consistent with family studies that have employed hierarchical diagnoses
(5,
8) in showing that the RDC schizoaffective syndrome shares familial liability with both the schizophrenic and manic syndromes. Beyond this, the current study suggests that the schizoaffective syndrome shares genetic liability with the schizophrenic and manic syndromes. Furthermore, the model-fitting results imply that the genetic liability to the schizoaffective syndrome is entirely shared with the other two syndromes. In contrast, individual-specific environmental effects contributing to the schizoaffective syndrome do not appear to be shared with the other syndromes. Since the model-fitting analysis performed in this study required information about within-person comorbidity, these results cannot be directly extrapolated to studies that use a hierarchical diagnostic approach.
Limitations and Implications of the Results
It is not known to what extent the current results would apply to alternative definitions of the syndromes investigated. This limitation applies particularly to the schizoaffective syndrome, although this disorder, defined hierarchically, also shows evidence of familial co-aggregation with both schizophrenia and affective disorders on the basis of the DSM-III-R criteria
(24). Attempts to find genetically meaningful subtypes of schizoaffective disorder have been inconclusive
(10,
23).
The possible effects of including other classes of relatives and following up the twins for a longer period are unknown. Also, as in most other genetic studies of psychoses, most of the clinical data were not collected prospectively, which may have caused underestimation of the occurrence of psychotic syndromes, reducing the evidence of comorbidity. In addition, heritability estimates may be overestimated, e.g., in the presence of undetected dominance or epistasis, and underestimated, e.g., with assortative mating, variability in the quality of clinical information, and imperfect reliability of clinical raters. Finally, replication of these results in independent groups of subjects would be valuable.
The results of this study suggest that valuable information about the familial aggregation of psychotic symptoms will be gained if quantitative genetic investigations take account of within-person comorbidity of psychotic syndromes in addition to applying the traditional hierarchical diagnostic approach. These results also suggest that if further research identifies susceptibility loci that are involved in schizoaffective syndrome, defined nonhierarchically, they may also be susceptibility loci for the schizophrenic and manic syndromes. Such findings would support the suggestions of others that hints of shared genetic susceptibility have already emerged from molecular genetic studies
(23).