Emerging evidence suggests that the γ-aminobutyric acid (GABA) neurotransmitter system may contribute to the pathophysiology and treatment of major depression
(1). Reports demonstrating increased GABA
B receptor binding in rodent brains after chronic administration of various antidepressant drugs
(2) initially led to a GABA-ergic hypothesis of antidepressant drug action. Although later studies have yielded inconsistent results regarding antidepressant effects on GABA
B receptor binding
(3), the recent use of several anticonvulsant agents with GABA-enhancing properties in the treatment of mood disorders has renewed interest in GABA’s potential role in the treatment of mood disorders
(4).
Several lines of evidence suggest that major depression is associated with impairment of GABA-ergic function. Multiple studies have shown low GABA concentrations in the plasma and CSF of depressed individuals
(1). Consistent with these findings were the results of our study
(5) using proton magnetic resonance spectroscopy (
1H-MRS) that was technically constrained to the region of the occipital cortex, which demonstrated lower GABA concentrations in depressed patients than in healthy subjects. Preliminary results from a second study
(6) suggest that these occipital GABA concentrations are higher after a course of ECT. The aim of this study was to determine whether occipital cortex GABA concentrations are also higher in depressed patients after treatment with selective serotonin reuptake inhibitor (SSRI) agents.
Method
A total of 18 subjects meeting the DSM-IV criteria for major depressive disorder, confirmed by the Structured Clinical Interview for DSM-IV, participated in the study after providing written informed consent. Subjects with a history of alcohol or substance abuse or dependence within the 6 months before the study were excluded, as were any subjects with a lifetime history of bipolar disorder, psychotic disorder, or axis II disorder. Two of the subjects had a comorbid diagnosis of panic disorder, and one had a comorbid diagnosis of social phobia. No subject received psychotropic medications for at least 2 weeks before the first 1H-MRS session. After completing the initial 1H-MRS session, each subject initiated treatment with an SSRI agent.
Eleven subjects (seven male, four female) with a mean age of 39.2 years (SD=8.5) completed the study protocol. Five subjects were not included in the analysis because it was not possible to obtain acceptable-quality spectra on one of the two MRS studies for each patient, and two subjects were not included because they were lost to follow-up before the posttreatment MRS study. Of the final 11 subjects, eight received fluoxetine (mean dose=23.8 mg/day, SD=7.9), and three received citalopram (mean dose=26.7 mg/day, SD=4.7); five patients also received yohimbine in addition to the SSRI as part of a second study. No additional psychotropic medications were allowed during the study period. After receiving the SSRIs for at least 5 weeks (mean=8.6 weeks, SD=2.7), the patients returned for the posttreatment 1H-MRS session. Scores on the Hamilton Depression Rating Scale were obtained before both 1H-MRS sessions.
Occipital cortex GABA concentrations were determined according to the methods described by us elsewhere
(5,
7). Briefly, the studies were performed by using a 2.1-T Oxford magnet with a 1-m bore, equipped with a Bruker Avance spectrometer (Bruker Instruments, Billerica, Mass.). With an 8-cm radiofrequency surface coil tuned to the
1H-MRS frequency of 89.43 MHz, a 1.5×3×3-cm volume of interest was centered on the midline of the occipital cortex, 2 cm deep from the dura. Automated first- and second-order shimming was used to optimize B
0 homogeneity. Homonuclear editing of the C4 GABA resonance at 3.0 parts per million (chemical shift scale) was performed by using the J-editing pulse sequence described previously
(5,
7). Two subspectra of 128 scans each were subtracted to obtain a difference spectrum that isolates total GABA (combined measure of GABA and the GABA-containing dipeptide homocarnosine). Localization techniques included three-dimensional image-selected in vivo spectroscopy with outer volume suppression, selective excitation, and use of a surface spoiler coil. The spectral acquisition characteristics were as follows: repetition time, 3.39 seconds; echo time, 68 msec; sweep width, 1,500 Hz; and acquisition time, 510 msec. The integral of the GABA peak was adjusted for the macromolecular contribution and compared to the total creatine signal for absolute quantification.
The effects of SSRI treatment on GABA concentrations and Hamilton depression scale scores were evaluated by using paired t tests.
Results
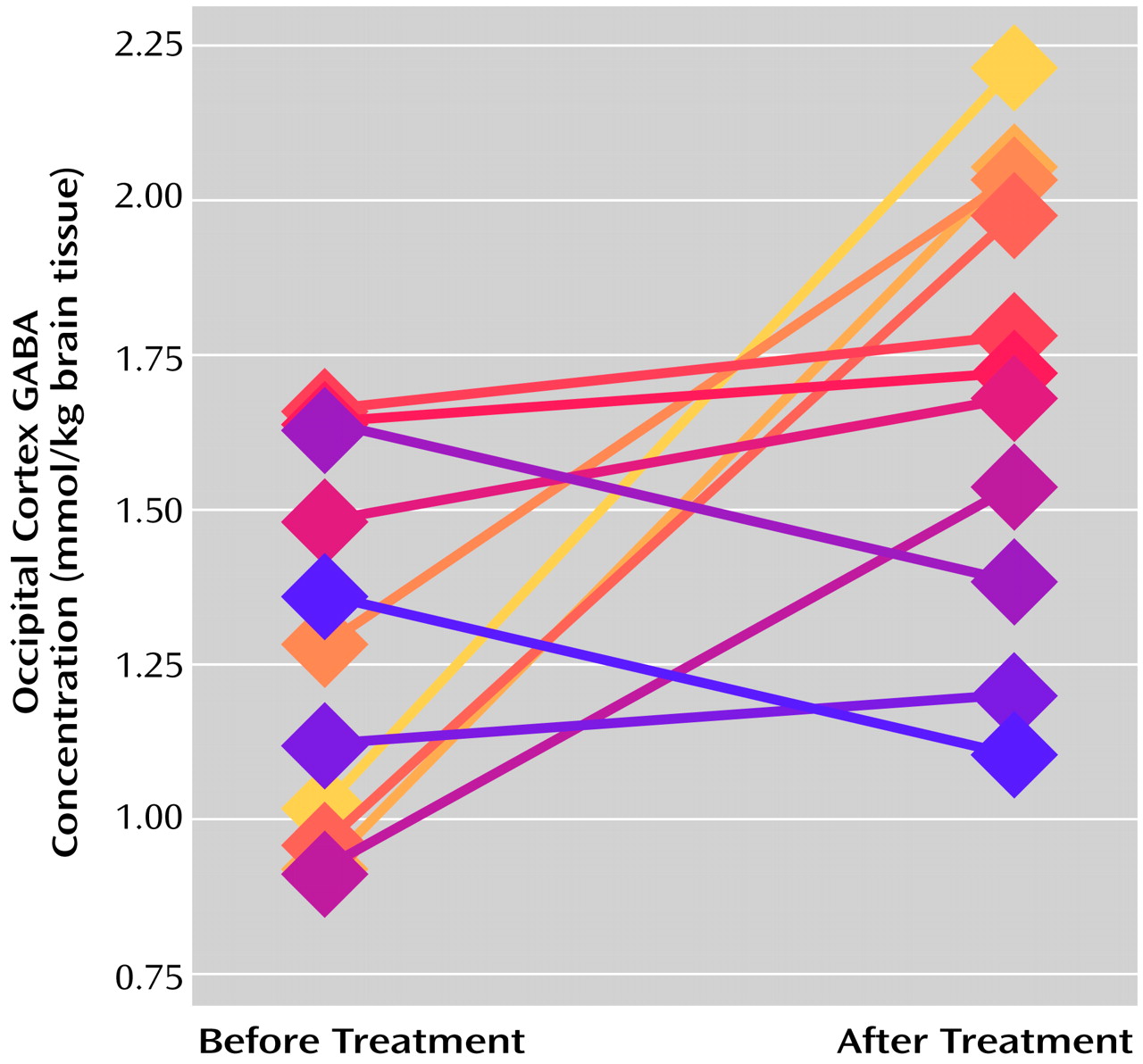
The occipital cortex GABA concentrations after treatment (mean=1.70 mmol/kg brain tissue, SD=0.37) were significantly higher than the pretreatment concentrations (mean=1.27, SD=0.30) (paired t test: t=2.61, df=10, p<0.03), and nine of the 11 subjects had higher posttreatment concentrations (
Figure 1). The posttreatment scores on the Hamilton depression scale (mean=9.73, SD=4.96) were significantly lower than the pretreatment scores (mean=26.00, SD=4.22) (paired t test: t=7.25, df=9, p<0.0001); all of the subjects had lower posttreatment scores, and seven of the 11 had reductions of more than 50%. No significant correlation was observed between the change in occipital cortex GABA concentration and clinical improvement, as determined by change in Hamilton depression score, in this small group (r=0.10, N=11, p>0.40). Nor was there a significant correlation between the pretreatment GABA concentration and clinical improvement (r=0.37, N=11, p>0.20). However, the five subjects with the lowest initial GABA concentrations (below 1.20 mmol/kg brain tissue) exhibited large increases in GABA concentration (mean change=0.88 mmol/kg brain tissue, SD=0.47) after SSRI treatment, whereas the five subjects with the highest pretreatment concentrations (above 1.35 mmol/kg brain tissue) showed no appreciable overall change in occipital GABA concentration after treatment (mean change=–0.02, SD=0.23). We did not observe a significant difference between the five subjects who also received yohimbine (mean=56% increase, SD=59) and the six subjects who received only SSRI treatment (mean=32% increase, SD=54) in this small study group (Mann-Whitney U=11.5, p>0.50).
Discussion
This pilot study showed a 34% increase in occipital cortex GABA concentrations after a course of SSRI treatment in a group of depressed patients. We previously demonstrated a similar increase in occipital GABA concentrations after a course of electroconvulsive therapy
(6). Together with earlier reports of low plasma, CSF, and cortical GABA concentrations in depressed subjects
(5,
8), these findings suggest that normalization of abnormally low cortical GABA concentrations may provide a common mechanism in the treatment of major depression.
The observed effects of the SSRI medications on occipital cortex GABA concentrations could be explained in part by the direct actions of serotonin (5-HT) on the GABA-ergic neurons in the cortex. The majority of serotonergic axons synapse on cortical GABA-ergic interneurons
(9). These GABA-ergic interneurons express either 5-HT
3 or 5-HT
2A receptors
(10) that can modulate activity in the short term and increase GABA release
(11). Studies by Mitoma and Konishi
(12) also demonstrate the ability of monoamines to elicit both short- and long-term enhancement of GABA-ergic inhibitory transmission through presynaptic mechanisms. However, the mechanism of increased presynaptic GABA-ergic transmission remains unclear since long-term administration of sertraline was found to produce either no change or a decrease in expression of glutamic acid decarboxylase in rats
(13).
The observation that subjects with the lowest pretreatment GABA concentrations have the largest increases after SSRI treatment is of additional interest. This may simply reflect a ceiling effect on occipital cortex GABA concentrations. Alternatively, it may suggest the existence of different subgroups of depressed patients, i.e., one group with abnormally low baseline GABA concentrations that exhibit large increases after treatment and a second with relatively normal baseline concentrations that show little change after treatment.
In conclusion, this pilot study suggests that like ECT, SSRI medications for the treatment of major depressive disorder result in increased occipital cortex GABA concentrations. This elevation of GABA concentrations after treatment may reflect either a direct action of the treatment on the GABA-ergic system or a reversal of a state-dependent phenomenon.